Abstract
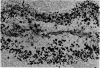
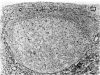
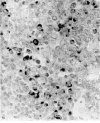
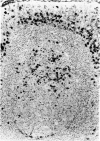
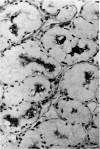
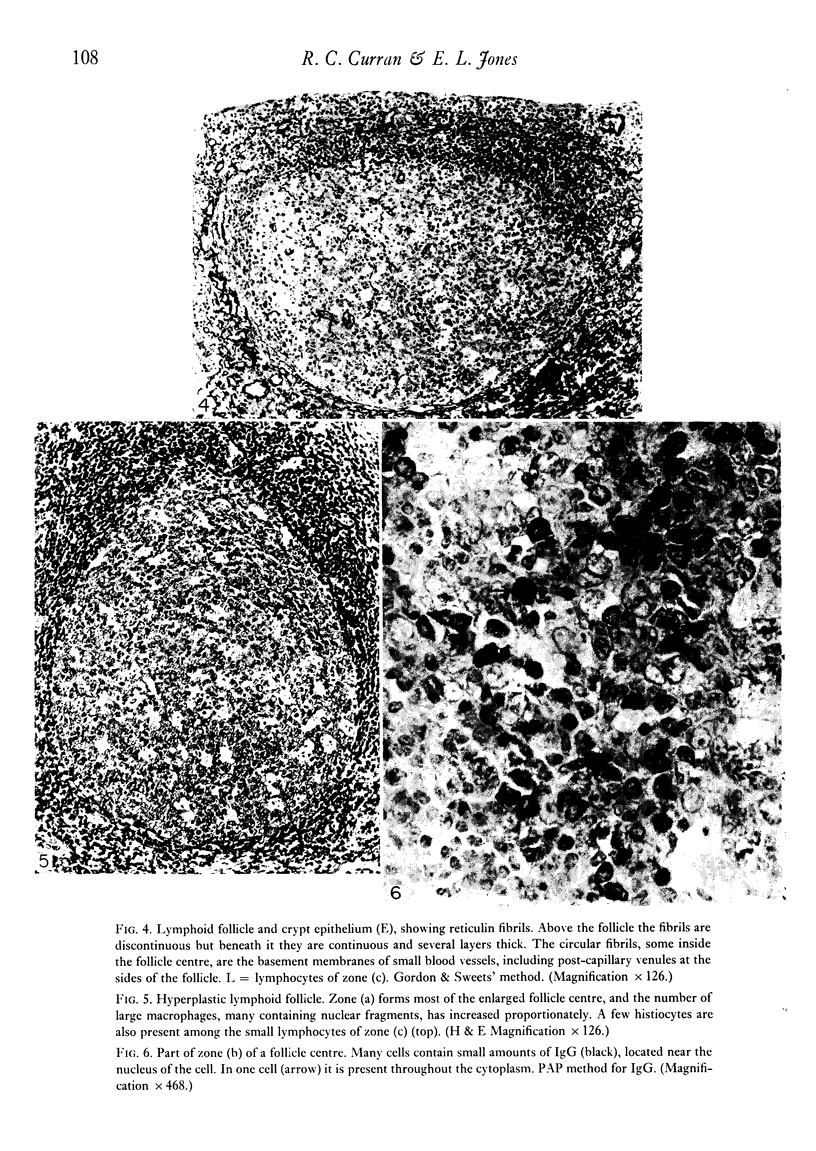
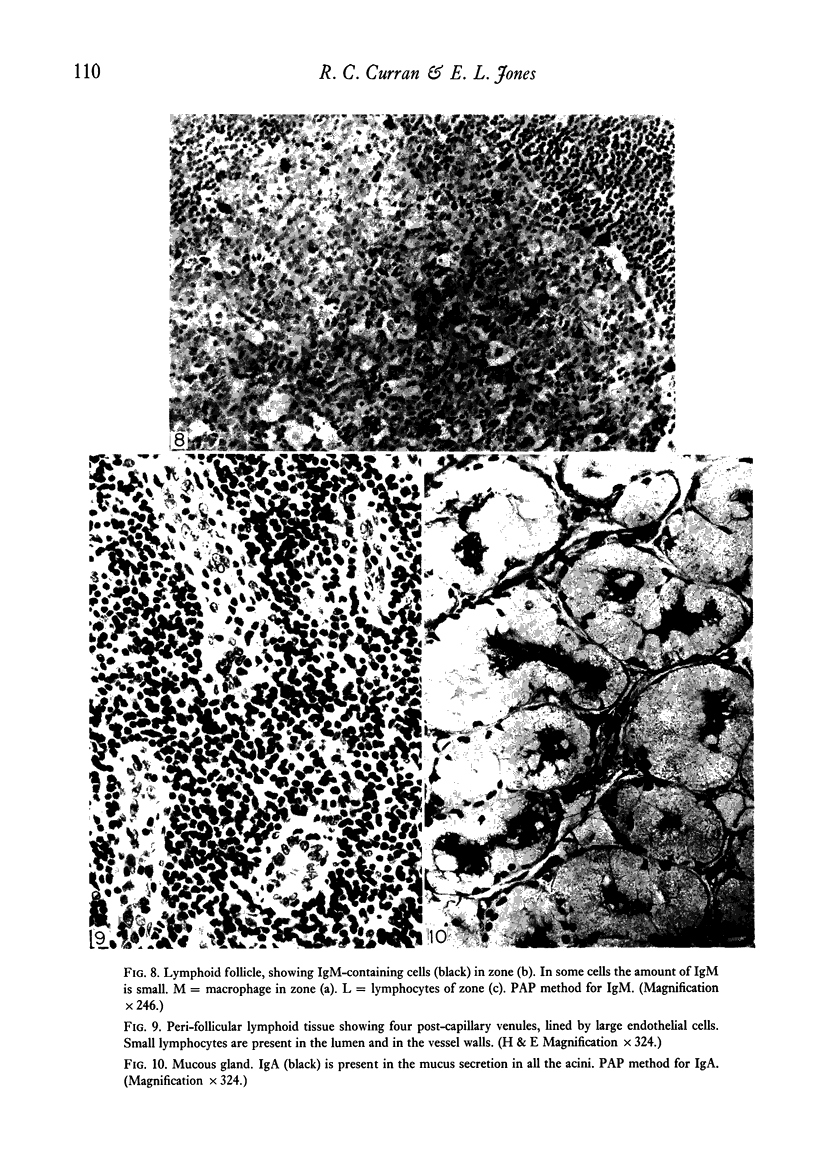
Intracellular immunoglobulin has been demonstrated in human palatine tonsils by the unlabelled antibody peroxidase-antiperoxidase complex (PAP) method in which rabbit antiserum to a range of human immunoglobulins (Igs) was linked to the PAP complex by an intermediate stage of swine antiserum to rabbit Ig. The effects of different methods of fixation and processing have been compared, formol-saline fixation giving the best results. The PAP technique proved greatly superior to the fluorescein isothiocyanate (FITC)-based technique, not only in sensitivity but in permitting study of the finer histological and cytological features. The lymphoid follicles are shown to have three distinct zones, two forming the follicle centre (zones (a) and (b)), and the third (zone (c)) the lymphocyte cap. Ig synthesis appeared to begin in the cells in zone (b). IgG, IgA, IgM, IgE and IgD were present in all tonsils, with IgG predominating, confirming that the tonsil resembles lymph nodes more closely than it does alimentary lymphoid tissue. Some follicles contained more than one type of Ig. The tonsil appears to have a well-developed T-dependent area, the lymphoid follicles forming a B-cell area. The structure of the tonsil would seem to facilitate contact between its lymphoid tissue and antigens in the crypts, and it is postulated that some T cells within the crypt epithelium, after contact with antigen, may leave the tonsil by the efferent lymphatics and enter the peripheral circulation by the thoracic duct, whilst other primed T cells interact with B cells in the follicle centres. Some B cells may then start to synthesize immunoglobulin, whilst others become memory cells in the lymphocyte 'cap' of the follicle.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burns J. Background staining and sensitivity of the unlabelled antibody-enzyme (PAP) method. Comparison with the peroxidase labelled antibody sandwich method using formalin fixed paraffin embedded material. Histochemistry. 1975 Jun 5;43(3):291–294. doi: 10.1007/BF00499711. [DOI] [PubMed] [Google Scholar]
- CAULFIELD J. B. Effects of varying the vehicle for OsO4 in tissue fixation. J Biophys Biochem Cytol. 1957 Sep 25;3(5):827–830. doi: 10.1083/jcb.3.5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRABBE P. A., CARBONARA A. O., HEREMANS J. F. THE NORMAL HUMAN INTESTINAL MUCOSA AS A MAJOR SOURCE OF PLASMA CELLS CONTAINING GAMMA-A-IMMUNOGLOBULIN. Lab Invest. 1965 Mar;14:235–248. [PubMed] [Google Scholar]
- Crabbé P. A., Heremans J. F. Distribution in human nasopharyngeal tonsils of plasma cells containing different types of immunoglobulin polypeptide chains. Lab Invest. 1967 Jan;16(1):112–123. [PubMed] [Google Scholar]
- Craddock C. G., Longmire R., McMillan R. Lymphocytes and the immune response. 2. N Engl J Med. 1971 Aug 12;285(7):378–384. doi: 10.1056/NEJM197108122850705. [DOI] [PubMed] [Google Scholar]
- Donovan R., Soothill J. F. Immunological studies in children undergoing tonsillectomy. Clin Exp Immunol. 1973 Jul;14(3):347–357. [PMC free article] [PubMed] [Google Scholar]
- Ferrarini M., Bargellesi A., Corte G., Viale G., Pernis B. Comparative study of membrane and intracytoplasmic immunoglobulin classes in human lymphoid cells. Ann N Y Acad Sci. 1975 Jun 30;254:243–253. doi: 10.1111/j.1749-6632.1975.tb29174.x. [DOI] [PubMed] [Google Scholar]
- GROCOTT R. G. A stain for fungi in tissue sections and smears using Gomori's methenamine-silver nitrate technic. Am J Clin Pathol. 1955 Aug;25(8):975–979. doi: 10.1093/ajcp/25.8_ts.0975. [DOI] [PubMed] [Google Scholar]
- Geha R. S., Schneeberger E., Rosen F. S., Merler E. Interaction of human thymus-derived and non-thymus-derived lymphocytes in vitro. Induction of proliferation and antibody synthesis in B lymphocytes by a soluble factor released from antigen-stimulated T lymphocytes. J Exp Med. 1973 Nov 1;138(5):1230–1247. doi: 10.1084/jem.138.5.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsbach A. B. The influence of different fixatives and fixation methods on the ultrastructure of rat kidney proximal tubule cells. I. Comparison of different perfusion fixation methods and of glutaraldehyde, formaldehyde and osmium tetroxide fixatives. J Ultrastruct Res. 1966 Jun;15(3):242–282. doi: 10.1016/s0022-5320(66)80109-0. [DOI] [PubMed] [Google Scholar]
- Mazurkiewicz J. E., Nakane P. K. Light and electron microscopic localization of antigens in tissues embedded in polyethylene glycol with a peroxidase labeled antibody method. J Histochem Cytochem. 1972 Dec;20(12):969–974. doi: 10.1177/20.12.969. [DOI] [PubMed] [Google Scholar]
- Mitchell J., Abbot A. Antigens in immunity. XVI. A light and electron microscope study of antigen localization in the rat spleen. Immunology. 1971 Aug;21(2):207–224. [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Gillespie D. N., Gleich G. J. Differences between IgA and IgE as secretory proteins. Clin Exp Immunol. 1975 Aug;21(2):306–317. [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Abbot A., Mitchell J. Antigens in immunity. XIV. Electron microscopic radioautographic studies of antigen capture in the lymph node medulla. J Exp Med. 1968 Feb 1;127(2):263–276. doi: 10.1084/jem.127.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Abbot A., Mitchell J., Lummus Z. Antigens in immunity. XV. Ultrastructural features of antigen capture in primary and secondary lymphoid follicles. J Exp Med. 1968 Feb 1;127(2):277–290. doi: 10.1084/jem.127.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse A. G., Polak J. M., Adams C., Kendall P. A. Diethylpyrocarbonate, a vapour-phase fixative for immunofluorescence studies on polypeptide hormones. Histochem J. 1974 May;6(3):347–352. doi: 10.1007/BF01312253. [DOI] [PubMed] [Google Scholar]
- Petts V., Roitt I. M. Peroxidase conjugates for demonstration of tissue antibodies: evaluation of the technique. Clin Exp Immunol. 1971 Sep;9(3):407–418. [PMC free article] [PubMed] [Google Scholar]
- Streefkerk J. G. Inhibition of erythrocyte pseudoperoxidase activity by treatment with hydrogen peroxide following methanol. J Histochem Cytochem. 1972 Oct;20(10):829–831. doi: 10.1177/20.10.829. [DOI] [PubMed] [Google Scholar]
- Tada T., Ishizaka K. Distribution of gamma E-forming cells in lymphoid tissues of the human and monkey. J Immunol. 1970 Feb;104(2):377–387. [PubMed] [Google Scholar]
- Taylor C. R., Burns J. The demonstration of plasma cells and other immunoglobulin-containing cells in formalin-fixed, paraffin-embedded tissues using peroxidase-labelled antibody. J Clin Pathol. 1974 Jan;27(1):14–20. doi: 10.1136/jcp.27.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE R. G. Antibody production by single cells. Nature. 1958 Nov 15;182(4646):1383–1384. doi: 10.1038/1821383a0. [DOI] [PubMed] [Google Scholar]
- White R. G., French V. I., Stark J. M. A study of the localisation of a protein antigen in the chicken spleen and its relation to the formation of germinal centres. J Med Microbiol. 1970 Feb;3(1):65–83. doi: 10.1099/00222615-3-1-65. [DOI] [PubMed] [Google Scholar]