Abstract
Clostridium thermocellum produces an extracellular multienzyme complex, termed cellulosome, that allows efficient solubilization of crystalline cellulose. One of the major enzymes in this complex is the CelS (Cel48A) exoglucanase. The regulation of CelS at the protein and transcriptional levels was studied using batch and continuous cultures. The results of sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analyses indicated that the amount of CelS in the supernatant fluids of cellobiose-grown cultures is lower than that of cellulose-grown cultures. The transcriptional level of celS mRNA was determined quantitatively by RNase protection assays with batch and continuous cultures under carbon and nitrogen limitation. The amount of celS mRNA transcripts per cell was about 180 for cells grown under carbon limitation at growth rates of 0.04 to 0.21 h−1 and 80 and 30 transcripts per cell for batch cultures at growth rates of 0.23 and 0.35 h−1, respectively. Under nitrogen limitation, the corresponding levels were 110, 40, and 30 transcripts/cell for growth rates of 0.07, 0.11, and 0.14 h−1, respectively. Two major transcriptional start sites were detected at positions −140 and −145 bp, upstream of the translational start site of the celS gene. The potential promoters exhibited homology to known sigma factors (i.e., σA and σB) of Bacillus subtilis. The relative activity of the two promoters remained constant under the conditions studied and was in agreement with the results of the RNase protection assay, in which the observed transcriptional activity was inversely proportional to the growth rate.
Clostridium thermocellum, a thermophilic, anaerobic, cellulolytic bacterium, produces an extracellular multienzyme complex termed cellulosome (2, 4, 7, 8, 14, 27, 43). This complex leads to an efficient solubilization of crystalline cellulose by numerous cellulases, which have endoglucanase and cellobiohydrolase (exoglucanase) activities. At present, more than 20 catalytically active subunits of the C. thermocellum cellulosome have been sequenced (6, 44). In addition to cellulases, there are also subunits that contain xylanase, mannanase, chitinase, and β-1,3-glucanase (lichenase) activities. In spite of these latter activities, C. thermocellum strain YS utilizes only cellulose and its degradation products (i.e., mainly cellobiose) as carbon sources (30, 38, 45). This bacterium was shown to grow on a limited number of other carbon sources (i.e., glucose, fructose, and sorbitol) but only after a relatively long adaptation period (21). It is likely that during this period, strains with mutations in their transport systems appear spontaneously with the ability to grow on the alternative carbon source (39).
Little is known how individual components of the complex are regulated. In an early work, it was shown that growth of C. thermocellum on cellobiose versus crystalline cellulose resulted in a different profile of cellulosomal components (5). In this context, the overall cellulase activity of cellulose-grown cells was shown to be higher than that of cellobiose-grown cells (5, 10, 11, 28). In another study, the transcriptional expression level of selected cellulosomal genes of a cellobiose-grown culture was examined. Theses genes included celA (cel8A), celD (cel9A), and celF (cel9B), and their transcripts could be detected not earlier than the late exponential phase, where cells presumably started to be limited on cellobiose (32). It was therefore suggested that the expression of cellulosomal cel genes was regulated by a mechanism analogous to catabolite repression. Transcriptional start site analysis of celD indicated involvement of sigma factors, based on homology to the consensus sequence of the σA and σD promoters of Bacillus subtilis. In this respect, a σD homologue has also been found in Clostridium acetobutylicum (42).
The previous works thus indicate that cellulosomal genes are affected by cellobiose concentration and/or phase of growth. In the present work, we studied the regulation of a critically important subunit, CelS (Cel48A), of the C. thermocellum cellulosome. This family 48 glycoside hydrolase is the most abundant cellulosomal enzyme subunit from this and other cellulosome-producing clostridia. CelS exhibits exoglucanase activity, and its activity is strongly inhibited by the presence of cellobiose (24, 25, 34, 36). The enzyme was sequenced (46), and its crystal structure was elucidated (17). In the present study, the regulation of expression of celS was studied under different physiological conditions, and the promoter region was characterized by primer extension analysis. The results established that this critical cellulosomal gene is regulated by growth rate.
MATERIALS AND METHODS
Organism, substrates, and culture conditions.
C. thermocellum YS was originally isolated from soil samples obtained at the hot springs of Yellowstone National Park (3, 28, 29). Cells were grown in batch culture at 60°C in Duran anaerobic bottles (Schott, Mainz, Germany), in medium containing the following ingredients (per liter): 0.65 g of K2HPO3 · 3H2O, 0.5 g of KH2PO4, 1.3 g of (NH4)SO4, 42 g of morpholinopropane sulfonic acid (MOPS), 5 g of yeast extract, 1 g of cysteine, 0.5 g of MgCl2, and 2 mg of resazurin. The medium included the desired carbon source (1.0%), either cellobiose from Acros Organics (Geel, Belgium) or microcrystalline cellulose (Avicel), obtained from Teva-Pharmaceutical Industries (Kfar Sava, Israel). Continuous cultures were performed in a BIOFLO 3000 fermentor (New Brunswick Scientific, Edison, N.J.) in a working volume of 1.5 liters at 60°C. A pH of 7.2 was maintained by automatic addition of 5 N NaOH. Agitation was kept constant at 100 rpm. To maintain anaerobic conditions, the headspace of the bioreactor contained 99.99% CO2 for initial growth and then changed to 99.99% N2 while starting continuous culture. Continuous cultures were operated under conditions of either cellobiose (2 g/liter) or nitrogen [0.04 g of (NH4)SO4 per liter] limitation, whereby C. thermocellum was adapted to different growth rates. It should be noted that C. thermocellum cannot utilize nitrogen or carbon from the yeast extract.
Preparation of extracellular material, cell-associated material, and purified cellulosome.
Extracellular (cell-free) material was obtained from the growth cultures by centrifuging the cells at 10,000 × g for 10 min. The supernatant fluids were collected and kept at −20°C in order to measure enzyme activity. When required, the supernatant fluids were concentrated by ultrafiltration using an Amicon PM10 membrane. Gel filtration was performed on either a Sepharose CL-4B column (1.3 by 77 cm) at room temperature, using a solution of 50 mM Tris-HCl buffer, 100 mM NaCl (pH 7.5), and 0.05% NaN3 at a flow rate of 0.5 ml/min, or on a Superose 6 column, using a fast protein liquid chromatography system (Pharmacia, Uppsala, Sweden). The samples were stored at −20°C for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analyses.
The precipitated cells (cells from 10 ml of culture in each tube) were washed twice with 10 mM Tris-HCl buffer (pH 7.5), and the pellets were snap-frozen in liquid nitrogen for RNA analyses and stored at −80°C.
N-terminal amino acid analysis.
For amino-terminal sequencing, cellulosome samples were subjected to SDS-PAGE and transferred to a polyvinylidene fluoride membrane (Applied Biosystems, Foster City, Calif.). The Coomassie blue-stained band was excised from the blot, and the amino-terminal sequence of the polypeptide was determined by Edman degradation (Applied Biosystems).
RNA extraction.
Total RNA was extracted from cells, using the RNeasy kit (Qiagen GmbH, Hilden, Germany) with minor modifications. Pellets of about 109 cells were suspended in 200 μl of lysis buffer (30 mM Tris-HCl [pH 7.5], 100 mM NaCl, 5 mM EDTA, 1% SDS). After incubation at 37°C for 30 min, the samples were sonicated (sonicator model W-375; Heat System-Ultrasonics Inc.) and centrifuged for 2 min at 12,000 × g, and the supernatant was loaded onto the column supplied in the kit. The remainder of the procedure was performed as recommended by the manufacturer. The concentration of RNA was determined by measuring A260, and the resultant RNA preparations were stored in aliquots at −80°C after snap-freezing in liquid nitrogen.
RPA.
The RNase protection assay (RPA) was performed using an RPAII kit (Ambion Inc., Austin, Tex.). Different amounts (0.5 to 3 μg) of RNA were hybridized with 32P-labeled antisense probe, and the protected RNAs were placed directly in a scintillation counter for quantification. The material was then separated on 5% polyacrylamide gels containing 7 M urea and visualized using a phosphorimager system. Labeling was performed by a Maxiscript in vitro transcription kit (Ambion), using T7 RNA polymerase. Transcription was accomplished using a construct containing a DNA fragment of the celS gene (spanning from −380 bp to + 48 bp relative to the initial ATG site) cloned into pBluescript II KS(+) (Stratagene, La Jolla, Calif.).
Northern blotting.
Northern hybridization of celS mRNA was performed by the method of Sambrook and Russell (41). The RNA was denatured in 50% formamide and 6% formaldehyde, and 15 μg of total RNA was loaded and subjected to electrophoresis on a 1% agarose gel containing 6% formaldehyde. The separated RNA was blotted onto a nitrocellulose membrane (Schleicher & Schuell, Inc., Keene, N.H.). The radioactive probe was prepared by random primer labeling, using the construct described above for RPA.
Determination of the amount of RNA per cell.
The amount of RNA was established for each of the cultures in which the transcript level of celS was determined. The pellet derived from a 30-ml culture was washed in 10 mM Tris-HCl (pH 7.6) and resuspended in 10 ml of 10% cold trichloroacetic acid (TCA). The suspension was kept on ice for 30 min. The supernatant fluids were discarded after 10 min of centrifugation at 10,000 × g. Cells were then resuspended in 5% TCA and centrifuged, and the pellet was dissolved gently in 1.5 ml 0.1 N NaOH using a sealed Pasteur pipette. The samples were incubated overnight at 37°C to allow complete hydrolysis of RNA. The solution was neutralized by 1 ml of 10% TCA, incubated for 15 min on ice, and centrifuged. To determine the RNA concentration in the sample, 1 volume of appropriately diluted supernatant fluid was mixed with 1 volume of orcinol reagent (1% orcinol dissolved in 0.1% FeCl3 in concentrated HCl), and the solution was boiled for 15 min. After the tubes were cooled under running tap water, 2 volumes of distilled water were added. The absorbance was recorded at two wavelengths, 600 nm (background) and 660 nm (background plus green complex). To determine the concentration of RNA or nucleotide, a deoxyadenosine solution of 50 μg/ml was used as a standard. Since the results are obtained as purine riboside equivalents of RNA, the values were doubled to obtain nucleotide equivalents (purines plus pyrimidines) and multiplied by the average nucleotide molecular weight. To determine the amount of total RNA per cell per culture, cells were counted using a Petroff-Hausser counting chamber.
Primer extension.
Primer extension with reverse transcriptase (avian myeloblastosis virus reverse transcriptase in the presence of RNasin RNase inhibitor [Promega, Madison, Wis.]) was performed according to the manufacturer's instructions. A synthetic oligonucleotide probe (5′-TTCTCTCCATCTTCCCC-3′) was end labeled by T4 polynucleotide kinase (Fermentas, Hanover, Md.) and hybridized to a complementary coding region downstream of the ATG translation start site of celS. A 50-μg sample of mRNA, obtained from the desired cell culture, was heated for 1 min at 90°C and hybridized for 2 min at 60°C with approximately 500,000 cpm of the labeled probe. Extension with reverse transcriptase was performed essentially by the method of Moran (37). Products of primer extension were analyzed on 6% acrylamide-8 M urea sequencing gel, together with sequencing reactions derived from the same oligonucleotide.
Enzyme assay.
Carboxymethyl cellulase (CMCase) activity was determined using a 1-ml assay mixture which contained 4% carboxymethyl cellulose (Sigma Chemical Co., St. Louis, Mo.) dissolved in 50 mM acetate buffer (pH 5) and 50 mM CaCl2. To this mixture, 5 μl of a cellulosome preparation (diluted appropriately) was added, and the samples were incubated at 60°C. At predetermined time intervals, 0.1-ml samples were examined for the presence of reducing sugars by the dinitrosalicylic acid procedure (31). Amorphous cellulase (28) and crystalline cellulase (22) activities were assayed in an identical manner, using 1% of the appropriate substrate, i.e., acid-swollen cellulose and microcrystalline cellulose (Avicel), respectively.
Miscellaneous methods.
SDS-PAGE was performed as described earlier (26). Immunoblotting was performed by the method of Morag et al. (36). Protein concentration was estimated spectrophotometrically (12).
RESULTS
Effect of carbon source on the cellulosomal subunit profile.
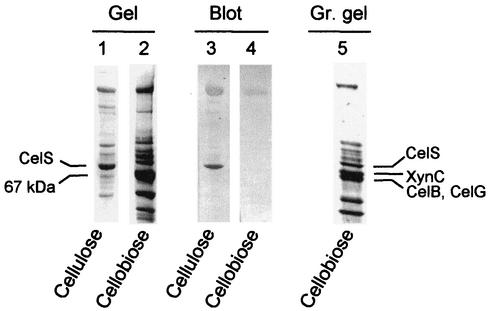
Previous results showed that the composition of the extracellular cellulosome differed as a function of the growth conditions (5, 23). In order to examine this phenomenon further, the cellulosome obtained from the supernatant of cultures grown on cellobiose to the late stationary phase (after 72 h of growth) was compared with that derived from cellulose-grown cells. The most prominent difference as revealed by SDS-PAGE analysis was a dramatic reduction of the CelS band in cellobiose-grown cells compared to cellulose-grown cells. In contrast, an enhancement of a 67-kDa band was observed upon growth on cellobiose (Fig. 1).
FIG. 1.
Western blot analysis of cellulosome preparations, purified from cellulose- and cellobiose-grown cells. Cellulosomes, derived from the late stationary phase of C. thermocellum, grown either on microcrystalline cellulose (lanes 1 and 3) or on cellobiose (lanes 2, 4, and 5), were isolated from the culture medium by affinity chromatography on cellulose. The preparations were separated on SDS-6% polyacrylamide gels (1, 2). Identical samples were transferred electrophoretically to nitrocellulose membranes and labeled with anti-CelS antibodies (3, 4). A similar cellulosome preparation, derived from cellobiose-grown cells harvested in the mid-exponential phase of growth, was separated on 7 to 12% gradient (Gr.) gel (5). The cellulosomal subunits XynC, CelB, and CelG were identified from polyvinylidene fluoride blots of the latter gel by amino acid sequence analysis.
It was important to verify that the CelS subunit was indeed lacking from the cellulosome derived from cellobiose-grown cells, and not present as a truncated version in the 67-kDa band. The cellulosome preparations were thus subjected to immunoblotting with anti-CelS. CelS was found to be the major component of the cellulosome from cells grown on crystalline cellulose (Fig. 1). In contrast, CelS was almost completely lacking from cellulosomes of cellobiose-grown cells (Fig. 1). Furthermore, the 67-kDa band was not detected by anti-CelS, indicating that this band is not a truncated version of CelS. The anti-CelS antiserum displays minor cross-reactivity with the scaffoldin, which helps with the unequivocal localization of CelS on the blot.
Characterization of the 67-kDa band.
In early experiments, xylanase activity was demonstrated to be associated with the cellulosomal S9 or S10 subunit (35). A primary candidate for one of the subunits, based on previously published immunochemical evidence and sequence data, is a known cellulosomal xylanase, XynC (1, 19). In fact, the bands referred to as the S9 and S10 subunits represent at least two proteins. It was thus necessary to clarify the identity of the proteins (i.e., cellulosomal enzymes) associated with these overlapping bands. Consequently, cellulosomal proteins, derived from the exponential phase of cellobiose-grown cells, were separated by 7 to 12% polyacrylamide gradient SDS-PAGE. Two bands could be discerned in the region of the 67-kDa band (Fig. 1). The identification of the upper band was determined by N-terminal amino acid sequence analysis, in which the first 12 residues (AALIYDDFETGL) were indeed found to be identical to those of XynC, a family 10 glycoside hydrolase. The lower band was subjected to chymotrypsin digestion, whereby two resultant peptides were sequenced (DYPINLGK and MWIDTLVWLADK), thus enabling the identification of two additional cellulosomal subunits, CelB and CelG, both of family 5 glycoside hydrolase. From the known sequences, the calculated molecular weights for the three subunits XynC, CelB, and CelG were 66,147, 61,063, and 59,769, respectively, results that are reasonably consistent with the observed banding profile.
Enzymatic activities of the cellulosomes.
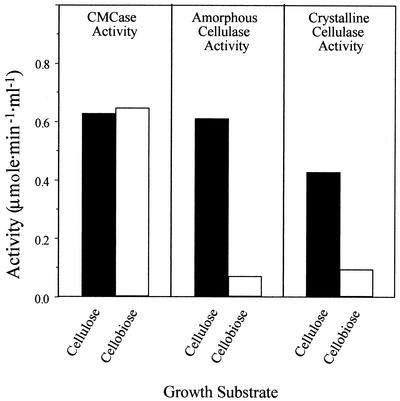
The enzymatic activities of the different cellulosome preparations were examined (Fig. 2) to determine whether the observed differences in enzyme composition are reflected in the overall action on cellulosic substrates. Cellulosomes prepared from cellulose-grown cells exhibited 3- to 10-fold-higher activities on amorphous cellulose and crystalline cellulose than cellulosomes prepared from cellobiose-grown cells. The differences in crystalline cellulase activities can be explained partly by the changes in the levels of CelS contained in the complex derived from the cellulose- versus cellobiose-grown cells as observed by SDS-PAGE (Fig. 1). In contrast, the two types of cellulosomes exhibited similar levels of endoglucanase (CMCase) activity, although the levels of the CelB and CelG endoglucanases are higher in cellulobiose-grown cells. These results may indicate significant differences in the cellulosome composition upon growth on cellobiose versus cellulose and the important role of CelS in the degradation of crystalline cellulose as suggested earlier.
FIG. 2.
Activity profiles of cellulosomes derived from cells grown on cellulose or cellobiose using different cellulosic test substrates. Equivalent amounts of the cellulosome preparations were examined on the indicated substrates (see Materials and Methods for details).
Expression of celS at the transcriptional level.
Our initial observations (exemplified in Fig. 1) indicated that the level of expression of CelS varied during the growth phase of cellobiose-grown cells. Higher amounts of cellulosomal CelS were obtained in mid-exponential phase, whereas in the late stationary phase of growth, no CelS could be detected (Fig. 1, lanes 5 and 2). However, studying the regulation of the cellulosomal genes at the protein level is problematic, since the cellulosome complex is present in both cell-free- and cell-bound forms. In cellulose-grown cells, at least part of the extracellular cellulosome fraction is absorbed to its substrate. Therefore, to determine the influence of carbon source or growth phase on the expression of celS, further investigations were performed at the transcriptional level. The celS mRNA level was assessed, first in batch cultures, grown either on cellobiose or microcrystalline cellulose during the course of growth.
Aliquots of C. thermocellum were removed at two different points on the growth curve, when cell cultures reached turbidities (A660) of 0.7 and 1.8 while growing on cellobiose and turbidities of 0.8 and 2.0 while growing on crystalline cellulose (Avicel). Turbidities of 0.7 and 0.8 are consistent with exponential growth, and turbidities of 1.8 and 2.0 are consistent with the late exponential or early stationary phase. Since cellulose is an insoluble substrate, the cultures were first subjected to vigorous vortexing and centrifuged at 100 × g for 1 min to remove the substrate before measuring turbidity. The maximum growth rates achieved for growth on cellobiose and cellulose were 0.35 and 0.23 h−1 (i.e., doubling times of 2 and 3 h), respectively.
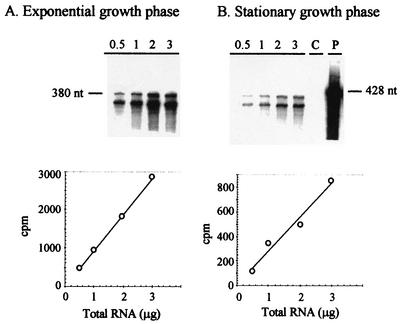
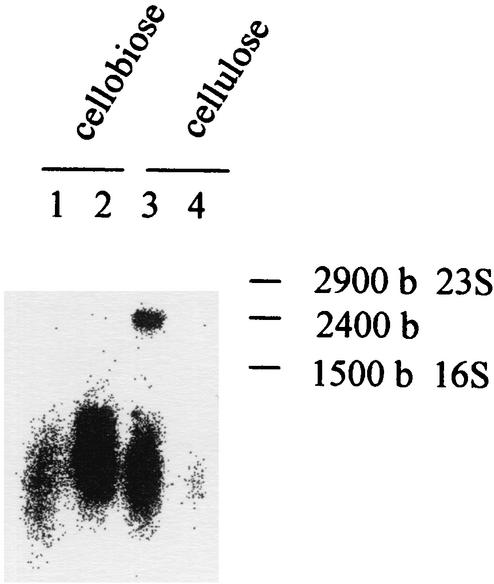
mRNA levels were measured by RPA using a 32P-labeled antisense RNA probe. The antisense probe was designed to include transcribed and untranscribed portions of the promoter region. In this way, the anticipated protected product would be smaller than the full-length probe, allowing simple verification that chromosomal DNA is not involved in the protection assay. Total RNA was thus extracted from the various samples, and different amounts of RNA were hybridized with the 32P-labeled celS antisense probe. The hybridized sample was then digested with an RNase cocktail, and after precipitation, the radioactivity of the protected RNA product was measured in a scintillation counter. Subsequently, the RPA products were separated on polyacrylamide gels containing 7 M urea and visualized using phosphorimager autoradiography (Fig. 3). Based both on the total count and the intensity of the bands obtained from the autoradiograph, the radioactive signal increased linearly with the increase in total RNA within the range of 0.5 to 3 μg. From these values combined with the specific activity of the probe and the amount of total RNA used for hybridization, the number of celS transcripts could be determined for 1 μg of total RNA. Data were obtained in two independent experiments for each set of conditions, and an average value of 1.8 × 10−7 μg of total RNA/cell ± 25% was obtained. This value was used to calculate the number of celS mRNA copies per cell for the different conditions used in this study. Higher levels of celS transcripts were obtained during exponential growth on microcrystalline cellulose. These levels were approximately threefold higher than those for exponential growth on cellobiose (i.e., 81 versus 28 transcripts per cell). In the cellulose-grown cell cultures, a dramatic reduction of the transcript levels was observed upon entry to the stationary phase (25 transcripts per cell). The reduction was less marked in cellobiose-grown cultures (20 transcripts per cell). To verify that the antisense RNA probes did indeed interact with the expected transcribed genes, a DNA-labeled probe was used (coding for the mRNA antisense probe) in Northern blot analysis (Fig. 4). The mRNA transcript of a size consistent with that of celS was labeled (2,400 bases). As can also be seen from Fig. 4, the observed transcript levels of celS for the different cell samples are consistent with the RPA results.
FIG. 3.
RPA for celS-initiated mRNA derived from cellulose-grown C. thermocellum cells. RPAs were performed using RNA from exponential- or stationary-phase cultures grown on crystalline cellulose. Cell samples were harvested and snap-frozen in liquid nitrogen, and total RNA was extracted using the RNeasy kit from Qiagen. Different amounts of RNA (indicated in micrograms above each lane) were hybridized overnight with 50,000 cpm of 32P-labeled 428-nt antisense celS probe, and then digested with RNase A-RNase T1 (Promega) for 30 min. The protected RNAs were placed directly in a scintillation counter for quantification and then separated on a 5% polyacrylamide gel containing 7 M urea. The radiolabeled bands were visualized using a phosphorimager system. The expected size of the protected products was 380 nt. (Top) Representative autoradiograph of the protected products subjected to phosphorimager analysis. (Bottom) Correlation between the amount of total RNA used in the assay and the counts obtained for the protected products. The negative control (lane C) contained yeast RNA instead of C. thermocellum RNA. The full-length probe was used in lane P. The graphs represent the average values of at least three separate experiments, and the experimental error was ±15%.
FIG. 4.
Expression of celS mRNA in C. thermocellum under different physiological conditions as determined by Northern blot analysis. Total RNA was isolated from cultures grown on either cellobiose or crystalline cellulose. The isolated total RNA (15 μg) was separated electrophoretically on an agarose gel and transferred to a nitrocellulose membrane. The resultant blot was hybridized with the celS DNA probe. The sizes of the rRNA (16S and 23S) and the estimated size of the transcript are indicated (in bases [b]).
Transcript level of celS in a chemostat with limited cellobiose.
When C. thermocellum was grown in batch culture on cellobiose or cellulose, the observed difference in celS expression was reflected in the different growth rates of the two cultures. The influence of growth rate on celS expression was investigated further in continuous culture.
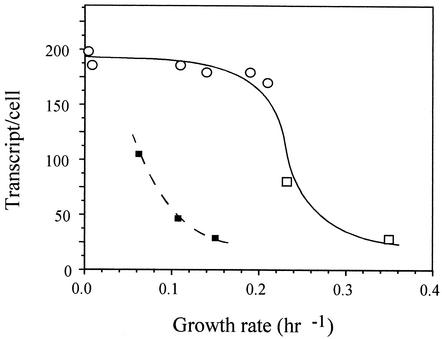
Continuous culture allowed us to examine the expression of celS under defined steady-state conditions and to study the influence of nutrient limitation (cellobiose or nitrogen) under conditions of different growth rates. Fresh medium was introduced into the fermentor at the appropriate dilution rate between 0.21 to 0.04 h−1. At dilution rates higher than 0.21 h−1, the cells were washed out of the fermentor, as noted previously (45). The establishment of steady-state conditions was assumed when the culture had been growing for a period of at least 3 generations in which the cell density (monitored spectrophotometrically) remained unchanged for at least 1 generation. Under conditions of cellobiose limitation (i.e., cellobiose added at a concentration of 2 g/liter), the turbidities were in the range of 0.5 to 0.7 A660. The transcript level of celS at a dilution rate of 0.21 h−1 was more than double that of cellulose-grown cells in batch culture (exponential phase) at a growth rate of 0.23 h−1 (170 versus 80 transcripts/cell, respectively). Additional decreases in the dilution rate did not lead to a significant increase in the transcript level of this gene. These data indicate that the expression of celS is influenced by growth rate until an observed rate of 0.21 h−1. Since the change in dilution rate in continuous culture indirectly reflects cellobiose concentration, we examined the influence of growth rate under nitrogen limitation and excess cellobiose.
Transcript level of celS in a chemostat with limited nitrogen.
Continuous cultures with limited nitrogen revealed that the expression of celS increases with reductions in the growth rate (Fig. 5), similar to that observed above for cultures subjected to cellobiose limitation. In this case, the level of celS increased more than threefold (100 versus 30 transcripts/cell) when the growth rate decreased from 0.14 to 0.07 h−1. However, under conditions of nitrogen limitation, a downshift was observed in the overall level of celS expression.
FIG. 5.
Transcript level of celS as a function of growth rate. The amount of celS mRNA was determined by RPA and given as number of transcripts per cell based on the average measured amount of total RNA in a single cell, 1.8 × 10−7 μg/cell. The values are an average of several measurements at an accuracy of ±25%. The cells were grown under the following conditions: continuous cultures under carbon (cellobiose) limitation (open circles), batch cultures on cellobiose or cellulose (open squares), and continuous cultures under nitrogen limitation (small black squares).
Primer extension analysis.
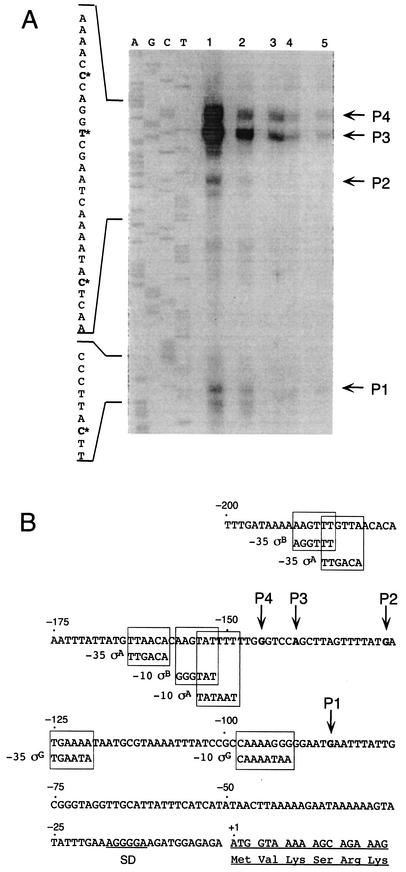
To identify potential regulatory elements that are involved in the transcription of celS, the transcription start site was determined under the conditions described above. Total RNA was extracted from cells in the mid-exponential phase of growth, either on cellobiose or cellulose, or from a continuous culture under cellobiose limitation (dilution rate of 0.04 h−1), and the samples were subjected to primer extension analysis. An appropriate synthetic oligonucleotide probe corresponding to the complementary sequence of a celS gene segment downstream of the initial ATG site (nucleotides [nt] +102 to +118) was used. Two major transcription start sites were obtained at positions −140 and −145 bp upstream of the translational start site of celS. Potential promoters (−35 and −10) were identified upstream of the transcriptional celS start site (Fig. 6). These promoters exhibit homology to known sigma factors (i.e., σA and σB) of Bacillus subtilis, although the space between the −10 and −35 region is not the usual 17 bp. The relative activity of the two promoters remain constant under the conditions studied, and in agreement with the RPA results, the level of transcriptional activity was inversely proportional to the growth rate.
FIG. 6.
Mapping of the 5′ terminus of celS by primer extension analysis. (A) 32P-labeled oligonucleotide was hybridized to mRNA obtained from C. thermocellum grown under the following conditions: continuous culture limited on cellobiose, diluted to a rate of 0.04 h−1 (lane 1); exponential-phase culture grown on Avicel (lane 2); late-exponential-phase culture grown on crystalline cellulose (lane3); exponential-phase culture grown on cellobiose (lane 4); and late-exponential-phase culture grown on cellobiose (lane 5). Dideoxynucleotide sequence reactions were performed using the same primer employed in the reverse transcriptase reactions. The positions of the transcriptional start points are indicated by an asterisk on the inferred nontemplate strand sequence. The products of primer extension are indicated (P1 to P4). (B) Sequence data for the regulatory region. The respective transcriptional start points (P1 to P4) are indicated. The consensus B. subtilis σA, σB, and σG promoter sequences are framed at the homologous sites of the C. thermocellum sequence. The proposed Shine-Dalgarno (SD) site and the initiating ATG codon are indicated.
DISCUSSION
Our biochemical results confirm earlier reports that the family 48 cellobiohydrolase subunit, CelS, is the major component of the C. thermocellum cellulosome (3, 28, 29, 34, 36, 46-48). Our results also support a previous study (5), in which it was observed that growth of cells on cellobiose led to a dramatic decrease in the content of the CelS subunit and concomitant increases in other cellulosomal components. These components were determined in this work to represent XynC, CelG, and/or CelB. On the basis of these results, it would appear that the composition of cellulosomal components is regulated by the growth conditions. However, for numerous reasons, studying gene regulation of cellulosomal subunits at the protein level is problematic. For example, the various cellulosomal subunits exhibit similar levels and types of activities on similar substrates. In many cases, the molecular sizes are very similar and the protein subunits often migrate similarly on SDS-polyacrylamide gels. Many of the subunits share conserved regions in their primary structures (notably in their dockerin sequences) and thus exhibit a high degree of immunogenic cross-reactivity. Moreover, cellulosome preparations are characteristically heterogeneous in their subunit content and distribution among various cell-associated and extracellular fractions. It should also be taken into account that most of the previous studies were conducted in batch culture from which the cellulosome fraction was usually purified at the late stationary phase of growth. At this stage, the conditions in the medium are difficult to define, especially in the case of cells growing on a recalcitrant insoluble substrate, such as cellulose. Consequently, in order to more precisely monitor the regulation of celS, its expression was determined in this work at the transcriptional level and under different well-defined growth conditions.
The level of celS mRNA was measured in cell cultures grown on cellulose or cellobiose and sampled during the exponential phase or in the early stationary phase of growth. The expression of celS was found to be threefold higher in cellulose-grown cells than in cellobiose-grown cells at the mid-exponential phase of growth. The low level of the celS mRNA transcripts during growth on cellobiose is directly proportional to the low levels of “true cellulase activity” in cells grown on cellobiose, compared to cellulose as obtained from this and other studies (15, 18), thus suggesting the critical involvement of CelS in the breakdown of recalcitrant substrates. Interestingly, the transcript levels of celS decreased in the early stationary phase of cultures grown on insoluble or soluble substrates, in contrast to that reported for other cellulosomal genes, i.e., celA, celD, and celF (32), thus suggesting that the various cellulosomal genes are regulated differently.
Since cellulose is an insoluble polymeric substrate and cannot enter the microbial cell, it must first be degraded by extracellular cellulases to soluble saccharides (mainly cellobiose) which would then be assimilated into the cell. In this context, it appears that the major factor that distinguishes cellobiose- versus cellulose-grown cells is the immediate availability of the two substrates. The recalcitrant nature of cellulose as a substrate was reflected in the maximum growth rate (μ = 0.23 h−1), which was lower than that of cellobiose-grown cultures (μ = 0.35 h−1). The correlation between growth rate and cellulase production was indeed supported by an earlier study (21), in which enhanced levels of cellulase production were obtained with slow-growing cells (on either crystalline cellulose or during adaptation to growth on unnatural substrates, i.e., fructose or sorbitol). On the other hand, another study (39) that investigated the correlation between cellulase production and various energetic parameters failed to find an obvious relationship with growth rate.
Continuous culture of C. thermocellum under cellobiose-limiting conditions verified the connection between growth rate and expression of celS. Growth rates even lower than that observed for cellulose-grown cells were accompanied by further increases in the level of celS transcripts, until a critical rate of 0.21 h−1, below which maximum values for expression were achieved. In contrast, during continuous culture under nitrogen limitation, cellobiose is in excess, independent of growth rate. Nevertheless, celS transcription was also increased as a function of decreased dilution rate, thus indicating the importance of growth rate in celS regulation as opposed to cellobiose concentration per se. Interestingly, the relatively low expression of celS under conditions of nitrogen limitation is in accord with the reported reduced expression of extracellular proteins by another cellulosome-producing bacterium under such growth conditions (13, 16).
Primer extension analysis indicated two major transcription start sites for celS. Consensus sequences, homologous to known promoter sequences for sigma factors (σA and σB) from B. subtilis, were identified upstream of these sites. Both σA- and σB-like promoters were active in the transcription of celS under all conditions examined, and their activity increased proportionally with increased growth rate. In B. subtilis, σA participates in the initiation of transcription of most of the housekeeping genes (20, 40). A similar primary function may be evident in C. thermocellum, as suggested by the presence of homologous σA promoter sequences associated with celS, as shown here, and with other cellulosomal genes (9, 32). On the other hand, σB in B. subtilis is known to play a role in stress responses (33). However, unlike its presence in the celS promoter, σB appears to be lacking in the promoters of the other cellulosomal genes that have been characterized thus far (9, 32).
In conclusion, celS appears to be regulated at the transcriptional level, and its expression is modulated by growth rate both under conditions of cellobiose and nitrogen limitation. Such conditions also exist in the natural anaerobic thermophilic cellulose-containing ecosystem, which dictates slow rates of growth. During growth of C. thermocellum on recalcitrant cellulose substrates, the family 48 CelS subunit is considered to play an essential and central role in the synergistic action among the other cellulolytic components of the cellulosome complex. It appears that the repression of CelS biosynthesis would be energetically favorable to the cell, under conditions (e.g., elevated cellobiose concentration) that support high growth rate.
Acknowledgments
This research was supported in part by the Israel Science Foundation (grants 771/01, 446/01, and 250/99), the United States-Israel Binational Agricultural Research and Development Fund (BARD research grant 3106-99C), and by a grant from the United States-Israel Binational Science Foundation (BSF), Jerusalem, Israel. Additional support was provided by the Technion’s Otto Meyerhof Center for Biotechnology, established by the Minerva Foundation (Munich, Germany), and funds from the Technion-Niedersachsen Cooperation (Hannover, Germany).
REFERENCES
- 1.Ali, B. R., M. P. Romaniec, G. P. Hazlewood, and R. B. Freedman. 1995. Characterization of the subunits in an apparently homogeneous subpopulation of Clostridium thermocellum cellulosomes. Enzyme Microbiol. Technol. 17:705-711. [DOI] [PubMed] [Google Scholar]
- 2.Bayer, E. A., H. Chanzy, R. Lamed, and Y. Shoham. 1998. Cellulose, cellulases and cellulosomes. Curr. Opin. Struct. Biol. 8:548-557. [DOI] [PubMed] [Google Scholar]
- 3.Bayer, E. A., R. Kenig, and R. Lamed. 1983. Adherence of Clostridium thermocellum to cellulose. J. Bacteriol. 156:818-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayer, E. A., E. Morag, and R. Lamed. 1994. The cellulosome—a treasure-trove for biotechnology. Trends Biotechnol. 12:378-386. [DOI] [PubMed] [Google Scholar]
- 5.Bayer, E. A., E. Setter, and R. Lamed. 1985. Organization and distribution of the cellulosome in Clostridium thermocellum. J. Bacteriol. 163:552-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayer, E. A., L. J. W. Shimon, R. Lamed, and Y. Shoham. 1998. Cellulosomes: structure and ultrastructure. J. Struct. Biol. 124:221-234. [DOI] [PubMed] [Google Scholar]
- 7.Bayer, E. A., Y. Shoham, and R. Lamed. September 2001, latest update. Cellulose-decomposing prokaryotes and their enzyme systems. In M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleifer, and E. Stackebrandt (ed.), The prokaryotes: an evolving electronic resource for the microbiological community, 3rd ed., release 3.7. Springer-Verlag, New York, N.Y. [Online.] http://link.springer.de/link/service/books/10125/index.htm.
- 8.Béguin, P., and M. Lemaire. 1996. The cellulosome: an exocellular, multiprotein complex specialized in cellulose degradation. Crit. Rev. Biochem. Mol. Biol. 31:201-236. [DOI] [PubMed] [Google Scholar]
- 9.Béguin, P., M. Rocancourt, M.-C. Chebrou, and J.-P. Aubert. 1986. Mapping of mRNA encoding endoglucanase A from Clostridium thermocellum. Mol. Gen. Genet. 202:251-254. [DOI] [PubMed] [Google Scholar]
- 10.Bhat, K. M., P. W. Goodenough, E. Owen, and T. M. Wood. 1993. Cellobiose: a true inducer of cellulosomes in different strains of Clostridium thermocellum. FEMS Microbiol. Lett. 111:73-78. [Google Scholar]
- 11.Bhat, S., J. F. Kennedy, P. W. Goodenough, E. Owen, and M. K. Bhat. 1997. Effect of D-glucono-1,4-lactone on the production of CMCase, pNPCase and true cellulase by Clostridium thermocellum. Carbohydr. Polymers 34:95-99. [Google Scholar]
- 12.Bradford, M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 13.Desvaux, M., E. Guedon, and H. Petitdemange. 2001. Carbon flux distribution and kinetics of cellulose fermentation in steady-state continuous cultures of Clostridium cellulolyticum on a chemically defined medium. J. Bacteriol. 183:119-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felix, C. R., and L. G. Ljungdahl. 1993. The cellulosome—the exocellular organelle of Clostridium. Annu. Rev. Microbiol. 47:791-819. [DOI] [PubMed] [Google Scholar]
- 15.Freier, D., C. P. Mothershed, and J. Wiegel. 1988. Characterization of Clostridium thermocellum JW20. Appl. Environ. Microbiol. 54:204-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guedon, E., S. Payot, M. Desvaux, and H. Petitdemange. 2000. Relationships between cellobiose catabolism, enzyme levels, and metabolic intermediates in Clostridium cellulolyticum grown in a synthetic medium. Biotechnol. Bioeng. 67:327-335. [DOI] [PubMed] [Google Scholar]
- 17.Guimaraes, B. G., H. Souchon, B. L. Lytle, J. H. D. Wu, and P. M. Alzari. 2002. The crystal structure and catalytic mechanism of cellobiohydrolase CelS, the major enzymatic component of the Clostridium thermocellum cellulosome. J. Mol. Biol. 320:587-596. [DOI] [PubMed] [Google Scholar]
- 18.Halliwell, G., T. M. Philips, and N. Halliwell. 1995. Microcrystalline forms of cellulose as substrates for strains of Clostridium thermocellum and cellulase formation. Proc. Biochem. 30:243-250. [Google Scholar]
- 19.Hayashi, H., K. I. Takagi, M. Fukumura, T. Kimura, S. Karita, K. Sakka, and K. Ohmiya. 1997. Sequence of xynC and properties of XynC, a major component of the Clostridium thermocellum cellulosome. J. Bacteriol. 179:4246-4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarmer, H., T. S. Larsen, A. Krogh, H. H. Saxild, S. Brunak, and S. Knudsen. 2001. Sigma A recognition sites in the Bacillus subtilis genome. Microbiology 147:2417-2424. [DOI] [PubMed] [Google Scholar]
- 21.Johnson, E. A., F. Bouchot, and A. L. Demain. 1985. Regulation of cellulase formation in Clostridium thermocellum. J. Gen. Microbiol. 131:223-232. [Google Scholar]
- 22.Johnson, E. A., M. Sakojoh, G. Halliwell, A. Madia, and A. L. Demain. 1982. Saccharification of complex cellulosic substrates by the cellulase system from Clostridium thermocellum. Appl. Environ. Microbiol. 43:1125-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosugi, A., K. Murashima, and R. H. Doi. 2001. Characterization of xylanolytic enzymes in Clostridium cellulovorans: expression of xylanase activity dependent on growth substrates. J. Bacteriol. 183:7037-7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kruus, K., A. Andreacchi, W. K. Wang, and J. H. Wu. 1995. Product inhibition of the recombinant CelS, an exoglucanase component of the Clostridium thermocellum cellulosome. Appl. Microbiol. Biotechnol. 44:399-404. [DOI] [PubMed] [Google Scholar]
- 25.Kruus, K., W. K. Wang, J. Ching, and J. H. Wu. 1995. Exoglucanase activities of the recombinant Clostridium thermocellum CelS, a major cellulosome component. J. Bacteriol. 177:1641-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 27.Lamed, R., and E. A. Bayer. 1988. The cellulosome of Clostridium thermocellum. Adv. Appl. Microbiol. 33:1-46. [Google Scholar]
- 28.Lamed, R., R. Kenig, E. Setter, and E. A. Bayer. 1985. Major characteristics of the cellulolytic system of Clostridium thermocellum coincide with those of the purified cellulosome. Enzyme Microb. Technol. 7:37-41. [Google Scholar]
- 29.Lamed, R., E. Setter, and E. A. Bayer. 1983. Characterization of a cellulose-binding, cellulase-containing complex in Clostridium thermocellum. J. Bacteriol. 156:828-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamed, R., and J. G. Zeikus. 1980. Ethanol production by thermophilic bacteria: relationship between fermentation product yields of and catabolic enzyme activities in Clostridium thermocellum and Thermoanaerobium brockii. J. Bacteriol. 144:569-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller, G. L. R., W. E. Blum, and A. L. Burton. 1960. Measurements of carboxymethylcellulase activity. Anal. Biochem. 2:127-132. [Google Scholar]
- 32.Mishra, S., P. Béguin, and J. Aubert. 1991. Transcription of Clostridium thermocellum endoglucanase genes celF and celD. J. Bacteriol. 173:80-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mittenhuber, G. 2002. A phylogenomic study of the general stress response sigma factor sigmaB of Bacillus subtilis and its regulatory proteins. J. Mol. Microbiol. Biotechnol. 4:427-452. [PubMed] [Google Scholar]
- 34.Morag, E., E. A. Bayer, G. P. Hazlewood, H. J. Gilbert, and R. Lamed. 1993. Cellulase Ss (CelS) is synonymous with the major cellobiohydrolase (subunit S8) from the cellulosome of Clostridium thermocellum. Appl. Biochem. Biotechnol. 43:147-151. [DOI] [PubMed] [Google Scholar]
- 35.Morag, E., E. A. Bayer, and R. Lamed. 1990. Relationship of cellulosomal and noncellulosomal xylanases of Clostridium thermocellum to cellulose-degrading enzymes. J. Bacteriol. 172:6098-6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morag, E., I. Halevy, E. A. Bayer, and R. Lamed. 1991. Isolation and properties of a major cellobiohydrolase from the cellulosome of Clostridium thermocellum. J. Bacteriol. 173:4155-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moran, C. P. 1990. Measuring gene expression in Bacillus, p. 267-294. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. Wiley & Sons, Chichester, United Kingdom.
- 38.Ng, T. K., and J. G. Zeikus. 1982. Differential metabolism of cellobiose and glucose by Clostridium thermocellum and Clostridium thermohydrosulfuricum. J. Bacteriol. 150:1391-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nochur, S. V., A. L. Demain, and M. F. Roberts. 1990. True cellulase production by Clostridium thermocellum grown on different carbon sources. FEMS Microbiol. Lett. 71:199-204. [Google Scholar]
- 40.Price, C. W., M. A. Gitt, and R. H. Doi. 1983. Isolation and physical mapping of the gene encoding the major sigma factor of Bacillus subtilis RNA polymerase. Proc. Natl. Acad. Sci. USA 80:4074-4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 42.Sauer, U., A. Treuner, M. Buchholz, J. D. Santangelo, and P. Durre. 1994. Sporulation and primary sigma factor homologous genes in Clostridium acetobutylicum. J. Bacteriol. 176:6572-6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwarz, W. H. 2001. The cellulosome and cellulose degradation by anaerobic bacteria. Appl. Microbiol. Biotechnol. 56:634-649. [DOI] [PubMed] [Google Scholar]
- 44.Shoham, Y., R. Lamed, and E. A. Bayer. 1999. The cellulosome concept as an efficient microbial strategy for the degradation of insoluble polysaccharides. Trends Microbiol. 7:275-281. [DOI] [PubMed] [Google Scholar]
- 45.Strobel, H. J., F. C. Caldwell, and K. A. Dawson. 1995. Carbohydrate transport by the anaerobic thermophile Clostridium thermocellum LQRI. Appl. Environ. Microbiol. 61:4012-4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang, W. K., K. Kruus, and J. H. D. Wu. 1993. Cloning and DNA sequence of the gene coding for Clostridium thermocellum cellulase SS (CelS), a major cellulosome component. J. Bacteriol. 175:1293-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, W. K., K. Kruus, and J. H. D. Wu. 1994. Cloning and expression of the Clostridium thermocellum cellulase celS gene in Escherichia coli. Appl. Microbiol. Biotechnol. 42:346-352. [DOI] [PubMed] [Google Scholar]
- 48.Wu, J. H. D., and A. L. Demain. 1988. Proteins of the Clostridium thermocellum cellulase complex responsible for degradation of crystalline cellulose, p. 117-131. In J.-P. Aubert, P. Béguin, and J. Millet (ed.), Biochemistry and genetics of cellulose degradation. Academic Press, London, United Kingdom.