Abstract
Hormone-sensitive lipase (HSL) is known to mediate the hydrolysis not only of triacylglycerol stored in adipose tissue but also of cholesterol esters in the adrenals, ovaries, testes, and macrophages. To elucidate its precise role in the development of obesity and steroidogenesis, we generated HSL knockout mice by homologous recombination in embryonic stem cells. Mice homozygous for the mutant HSL allele (HSL−/−) were superficially normal except that the males were sterile because of oligospermia. HSL−/− mice did not have hypogonadism or adrenal insufficiency. Instead, the testes completely lacked neutral cholesterol ester hydrolase (NCEH) activities and contained increased amounts of cholesterol ester. Many epithelial cells in the seminiferous tubules were vacuolated. NCEH activities were completely absent from both brown adipose tissue (BAT) and white adipose tissue (WAT) in HSL−/− mice. Consistently, adipocytes were significantly enlarged in the BAT (5-fold) and, to a lesser extent in the WAT (2-fold), supporting the concept that the hydrolysis of triacylglycerol was, at least in part, impaired in HSL−/− mice. The BAT mass was increased by 1.65-fold, but the WAT mass remained unchanged. Discrepancy of the size differences between cell and tissue suggests the heterogeneity of adipocytes. Despite these morphological changes, HSL−/− mice were neither obese nor cold sensitive. Furthermore, WAT from HSL−/− mice retained 40% of triacylglycerol lipase activities compared with the wild-type WAT. In conclusion, HSL is required for spermatogenesis but is not the only enzyme that mediates the hydrolysis of triacylglycerol stored in adipocytes.
Hormone-sensitive lipase (HSL) is a multifunctional enzyme that catalyzes the hydrolysis of triacylglycerol stored in adipose tissue and cholesterol esters in the adrenals, ovaries, testes, and macrophages (1, 2). It is encoded by a gene located on chromosome 19, and lacks homology to members of the pancreatic lipase gene family such as lipoprotein lipase (LPL) (3).
The lipolytic activities of HSL are under acute neuronal and hormonal control. Catecholamines and other lipolytic hormones stimulate these activities through the reversible phosphorylation of serine by cyclic adenosine 3′,5′-monophosphate (cAMP)-dependent protein kinase (PKA) (4). Conversely, insulin, an antilipolytic hormone, suppresses its activities by preventing phosphorylation (5). Therefore, the activation of this enzyme in adipose tissues is thought to account for the elevation of plasma free fatty acid (FFA) levels in various conditions such as starvation or diabetes mellitus. Since mice lacking β3-adrenergic receptor (6), a dominant form of β-adrenergic receptor expressed in adipocytes, are obese and since mice lacking the RIIβ subunit of PKA are lean because of increased PKA activity (7), it is reasonable to speculate that HSL mediates the regulation of adiposity by the adrenergic signaling pathway.
In addition to the triacylglycerol lipase activity, neutral cholesterol ester hydrolase (NCEH) activity in the adrenals and ovaries is mediated by HSL (2, 8, 9), implicating its role in steroidogenesis. HSL is also expressed in testicular cells, including spermatids and spermatozoa (10–12), suggesting its involvement in sperm development and/or function. Furthermore, NCEH activities expressed in aorta and macrophage cell lines have similar hormone sensitivity to triacylglycerol lipase activities in adipocytes (13, 14). In addition, HSL overexpression stimulates NCEH activities in macrophages (15). Therefore, it is widely accepted that HSL is responsible for the NCEH activities in macrophages (16–18), although there are those who disagree (19). Paradoxically, however, transgenic mice overexpressing HSL under the control of scavenger receptor A promoter-enhancer are not protected against atherosclerosis (20).
In an attempt to elucidate the bona fide functions of HSL, we generated HSL knockout mice (HSL−/−). These animals were not obese and were as cold sensitive as wild-type mice. Unexpectedly, HSL−/− male mice were sterile because of oligospermia. These results indicate the critical role of HSL in spermatogenesis and the redundancy of lipases that catalyze the hydrolysis of triacylglycerol in adipose tissue.
Methods
Generation of the HSL Knockout Mice.
The targeting vector construct was generated by insertion of a 1.4-kb PstI fragment with sequences of intron 6 to intron 7 as a short arm and 12 kb of HSL genomic sequences including exons 1 to 5 as a long arm into the 3′ and 5′ sides of the pol2neobpA expression cassette, respectively (11). Two copies of the herpes simplex thymidine kinase (tk) gene were inserted in tandem at the 3′ end of the short arm. The resultant construct was linearized by SalI digestion and electroporated into JH-1 embryonic stem cells. Selection with G418 and 1-(2-deoxy-2-fluoro-β-d-arabinofuranosyl)-5-iodouracil (FIAU) was performed as already described (21). Twenty-five of 144 clones resistant to both G418 and FIAU harbored the desired product of homologous recombination as judged by Southern blotting, and they were injected into blastocysts to generate chimeras. Chimeric males born to three independent embryonic stem cell clones were crossed with C57BL/6 females, and they gave the germ-line transmission. Mice were maintained on a 12-h dark/light cycle and were fed a normal chow diet. Unless otherwise indicated, tissues were prepared from males between 9 and 14 weeks old.
Peritoneal Macrophages.
One milliliter of 5% thioglycolate broth was injected into the peritoneal cavities of mice. After 4 days, the peritoneal cavities were lavaged with 16 ml of ice-cold saline. The cells were washed three times with PBS and resuspended in DMEM to give a concentration of 106 cells per ml, and 10 ml per dish was plated in 10-cm dishes. After incubation at 37°C for 2 h, the nonadherent cells were removed by washing three times with warmed PBS. Cells were incubated in DMEM containing 10% FCS for 24 h and harvested for the experiments.
Northern Blot Analysis.
Ten micrograms of total RNA was subjected to 1% agarose gel electrophoresis in the presence of formalin. The fractionated RNA was transferred to Hybond N (Amersham Pharmacia). The filters were hybridized to 32P-labeled cDNA probes corresponding to exon 1 or 8 sequences.
Immunoblot Analysis.
Tissues were homogenized in buffer A (0.25 M sucrose/1 mM EDTA/2 μg/ml leupeptin/50 mM Tris⋅HCl, pH 7.0) and centrifuged at 100,000 × g for 45 min at 4°C. The supernatant was used for the immunoblot analysis, which used an enhanced chemiluminescence kit (ECL; Amersham) and rabbit anti-rat HSL antibody (12).
Assays for NCEH and Triacylglycerol Lipase Activity.
Tissues were homogenized in buffer A and centrifuged at 100,000 × g for 45 min at 4°C. The supernatant was used for the enzyme assay. Macrophages were sonicated in buffer A and used directly for the enzyme assay. NCEH activity was measured essentially as described by Hajjar et al. (13), using a reaction mixture containing 6.14 μM cholesterol [1-14C]oleate (48.8 μCi/μmol; 1 μCi = 37 kBq). Triacylglycerol lipase activity was measured according to a modified method of Hajjar et al. (13). In brief, the samples were incubated at 37°C for 30 min in a final volume of 200 μl of a reaction mixture containing 105 μM tri[3H]oleoylglycerol (99.4 μCi/μmol), 23.7 μM lecithin, 12.5 μM sodium taurocholate, 1 M NaCl, and 85 mM potassium phosphate, pH 7.0. The high concentration of NaCl was included to inactivate LPL, which is a major triacylglycerol lipase expressed by adipocytes.
Lipids and Hormones.
Cholesterol, triacylglycerol, and FFA were determined enzymatically with Determiner TC555, Determiner TG555, and nonesterified fatty acids (NEFA) (Kyowa Medics, Tokyo), respectively. Plasma glycerol levels were determined by Determiner TG555, and glycerol in the incubation media was determined by a radiometric assay according to Bradley and Kaslow (22). Plasma levels of luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone were determined by using radioimmunoassay kits (Amersham Life Science and Nippon DPC).
Adrenocorticotropic Hormone (ACTH) Stimulation Tests.
Blood was collected before and 1 h after the intraperitoneal injection of 25 μg of β1–24 ACTH (Cortrosyn, Organon). Corticosterone was measured by a radioimmunoassay (ICN Biomedicals).
Histology.
Tissues were fixed with neutral-buffered formalin and embedded in paraffin. Sections were stained with hematoxylin and eosin. Image capture and analysis were performed with Adobe PhotoShop 3 image analysis software. The contour of each adipocyte was traced by hand and the cytoplasmic area was determined.
Number and Motility of Sperm.
The number and motility of epididymal sperm were examined as described (23).
Tissue DNA and Lipids.
Tissues were digested in SNET buffer (1% SDS/400 mM NaCl/5 mM EDTA/20 mM Tris·HCl, pH 8.0) containing proteinase K (0.2 mg/ml). After extraction with phenol/chloroform, DNA concentrations were fluorimetrically determined with bisbenzimide H33258, using ultrapure calf thymus DNA (Sigma) as the standard. Tissue lipids were extracted from approximately 100 mg of tissues according to the method of Folch et al. (24) and then enzymatically measured.
Oxygen Consumption (VO2).
VO2 and respiratory quotients (RQ) were determined as previously described (25). Mice were fasted or fed for 24 h. BRL35135A, a β3 agonist, was given intraperitoneally to the mice at 1 μg/g of body weight.
Cold-Sensitivity Test.
Mice were placed in a chamber whose temperature was set at 4°C, and their core body temperature was monitored with a model 43 TA telethermometer and a model 402 colonic probe (Yellow Springs Instruments, Yellow Springs, OH) every 1 h (26).
In Vitro Lipolysis.
Adipocytes were isolated from epididymal fat pads of male mice (12–16 weeks old) by using collagenase digestion according to Rodbell's method (27). One hundred microliters of 10% (vol/vol) cell suspension was incubated in a final volume of 500 μl of a buffer containing 120 mM NaCl, 4 mM KH2PO4, 1 mM CaCl2, 10 mM NaHCO3, 30 mM Hepes (pH 7.4), 3% (wt/vol), BSA and 1 unit/ml adenosine deaminase, with or without 100 μM isoproterenol, at 37°C. Glycerol and FFA were measured over a 15-min period.
In Vivo Lipolysis.
After a 12-h fast, isoproterenol (0.3 mg/kg) or saline was injected intraperitoneally into control and HSL−/− mice 12–16 weeks old; 15 min later, blood was quickly collected from the retroorbital venous plexus without anesthesia essentially as described by Suslic et al. (6). Plasma was separated for the determination of glycerol and FFA.
Results
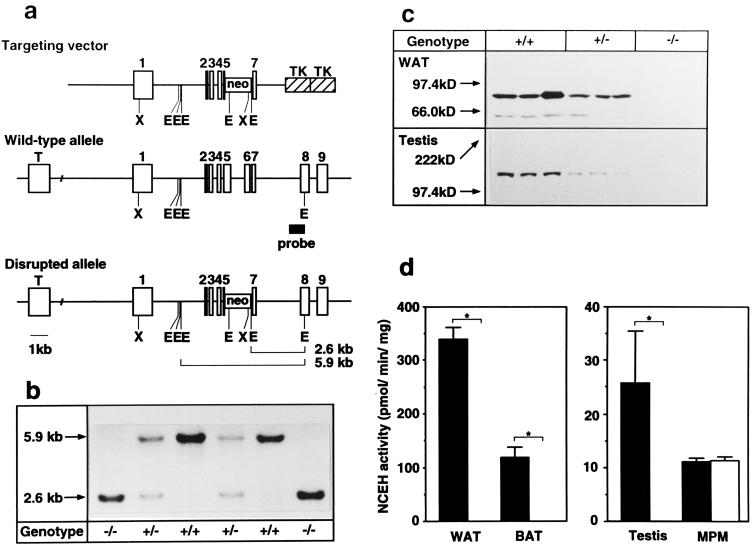
The gene encoding HSL was disrupted in embryonic stem cells by homologous recombination. A portion of exon 5, and the entire exon 6, which encodes the catalytic domain (3, 11, 28, 29), were replaced with a neo cassette (Fig. 1a). The intercross of the progeny (HSL+/−) resulted in offspring of both sexes with all three genotypes at the HSL locus with the expected Mendelian ratios (57:103:51; χ2 = 0.23, P > 0.05) (Fig. 1b). Northern blot analysis revealed that epididymal fat from HSL−/− mice lack a wild-type transcript with a size of ≈3.3 kb (data not shown). Western blot analysis showed no immunoreactive HSL protein in either epididymal fat (84 kDa) or testes (130 kDa) from HSL−/− mice (Fig. 1c). NCEH activities were undetectable in various organs, including WAT, BAT, and testes from the HSL−/− mice, but were not reduced in macrophages in culture (Fig. 1d).
Figure 1.
Targeted disruption of the HSL gene. (a) Map of the HSL gene and targeting construct. The targeting vector was designed to delete HSL genomic sequences including a part of exon 5 and the entire exon 6 encoding the catalytic motif (GXSXG). Targeted events generate an enzymatically inactive truncated protein lacking the C-terminal part of HSL. Tall boxes represent exons. The probe used for the diagnostic Southern blot is indicated as a shaded box. Restriction sites: X, XbaI; E, EcoRI. (b) Southern blot of EcoRI-digested DNA. The wild-type genomic fragment is 5.9 kb, whereas the mutated gene is 2.6 kb. Genotypes are indicated below each lane. (c) Immunoblot analysis of white adipose tissue (WAT) and testes, showing the absence of immunoreactive HSL in −/− mice. Note that the testicular isoform (HSLtes) is larger than the adipocyte isoform (HSLadi) (130 kDa vs. 84 kDa). Molecular masses of the standards are indicated on the left of the gel (5–15% acrylamide). (d) NCEH activities of WAT, brown adipose tissue (BAT) and testes were abolished in HSL−/− (open bars), whereas those of mouse peritoneal macrophages (MPM) elicited by thioglycolate were comparable to wild-type (solid bars). Enzyme activity is expressed as picomoles of substrate hydrolyzed per milligram of protein per minute. Data are represented as mean ± SE. Numbers of mice used are as follows: 4 +/+ and 4 −/− for WAT; 6 +/+ and 5 −/− for BAT; 3 +/+ and 3 −/− for testes; and 7 +/+ and 8 −/− for MPM.
When HSL−/− male mice were mated with female mice, they copulated normally, as evidenced by the formation of vaginal plugs, but none of the female mice mated to HSL−/− males became pregnant. HSL−/− mice had smaller testes than HSL+/+ mice (Table 1). Sperm counts, in epididymis preparations, revealed that HSL−/− mice contained only 94 ± 30 spermatozoa per epididymis, whereas HSL+/+ mice contained 7.7 ± 0.4 × 106 spermatozoa per epididymis. The sperm from the HSL−/− epididymis were not motile, whereas 60% of those from wild-type mice were motile. The thickness of the epithelial layers of HSL−/− seminiferous tubules was greatly reduced from 12 to 5–7 layers (Fig. 2). The number of mature spermatids was also markedly reduced. Extensive vacuolation was observed in the epithelial cells. The vacuoles may contain a cholesterol ester, since testicular cholesterol ester content was increased by 2.3-fold in the HSL−/− mice but the triacylglycerol content was not (Table 1). This observation is consistent with the finding that there was no difference in triacylglycerol lipase activities between wild-type and HSL−/− testes (38.6 ± 4.5 vs. 32.8 ± 6.5 nmol/h per mg; P > 0.05, n = 5). HSL−/− epididymis contains only degenerated spermatocytes and spermatids with a noticeable lack of mature sperms (Fig. 2). There were no detectable abnormalities in the interstitial cells, including the Leydig cells, in HSL−/− mice. Plasma levels of testosterone, follicle-stimulating hormone, and luteinizing hormone were not significantly different between the wild-type and HSL−/− mice, indicating that the oligospermia did not result from a hormonal insufficiency. Furthermore, plasma corticosterone levels were not different between the wild-type and HSL−/− mice before (143 ± 31 vs. 109 ± 18 ng/ml; P > 0.05, n = 8) or after stimulation with adrenocorticotropic hormone (460 ± 61 vs. 387 ± 17 ng/ml; P > 0.05, n = 8).
Table 1.
Triacylglycerol, free cholesterol, and cholesterol ester contents in testis, BAT, and WAT
| Tissue | Genotype | n | Weight, mg | Tissue lipids, mg/g
tissue
|
||
|---|---|---|---|---|---|---|
| TG | FC | EC | ||||
| Testis | +/+ | 5 | 128 ± 6 | 4.9 ± 1.6 | 9.4 ± 0.2 | 3.0 ± 0.4 |
| −/− | 5 | 86 ± 4* | 4.2 ± 0.9 | 10.2 ± 0.2 | 6.9 ± 1.2** | |
| BAT | +/+ | 4 | 103 ± 15 | 457 ± 15 | 0.13 ± 0.02 | 0.08 ± 0.01 |
| −/− | 4 | 170 ± 18** | 548 ± 27** | 0.04 ± 0.02** | 0.07 ± 0.01 | |
| WAT | +/+ | 4 | 356 ± 36 | 512 ± 137 | 0.07 ± 0.04 | 0.04 ± 0.03 |
| −/− | 4 | 277 ± 45 | 798 ± 75 | 0.19 ± 0.09 | 0.12 ± 0.06 | |
Tissue levels of total cholesterol (TC), free cholesterol (FC), and triacylglycerol (TG) were measured enzymatically. Esterified cholesterol (EC) levels were calculated from TC and FC values. Values are expressed as mean ± SE. *, P < 0.001; **, P < 0.05 versus HSL+/+ by Student's t test.
Figure 2.
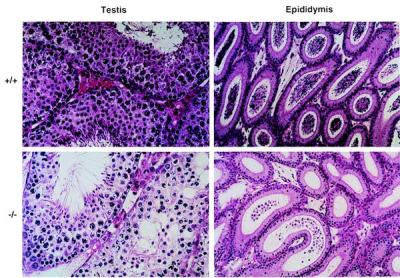
Histology of epididymis (×50) and testis (×100). HSL−/− epididymis contains no sperms but degenerated spermatocytes and spermatids. The thickness of the epithelial layers of the HSL−/− seminiferous tubules was greatly reduced from 12 to 5–7 layers. The number of mature spermatids was also markedly reduced. Extensive vacuolation was observed in the epithelial cells.
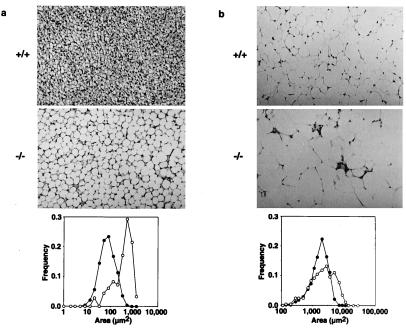
Because HSL is highly expressed in adipose tissues, we examined the weight and morphology of BAT and WAT. BAT was significantly enlarged in HSL−/− mice by 1.65-fold, primarily because of cellular hypertrophy, because triacylglycerol content was increased (Table 1) but DNA content was not (Table 2). In support of this observation, the median cytoplasmic area of BAT adipocytes was increased by about 5-fold in HSL−/− mice (Fig. 3a). Less remarkable cellular hypertrophy was observed in HSL−/− WAT, where the median cytoplasmic area was enlarged by 2-fold (Fig. 3b). Nevertheless, the WAT mass was not increased in the gonadal, retroperitoneal, or femoral regions (Table 2).
Table 2.
Tissue mass and DNA content of adipose tissues in various sites
| Tissue | Genotype | n | Fat mass, mg | Tissue DNA, μg | Mass/DNA × 103 |
|---|---|---|---|---|---|
| BAT | +/+ | 4 | 72 ± 3 | 183 ± 2 | 0.39 ± 0.02 |
| −/− | 4 | 139 ± 15* | 196 ± 20 | 0.71 ± 0.05* | |
| Epididymal WAT | +/+ | 4 | 371 ± 85 | 147 ± 17 | 2.44 ± 0.34 |
| −/− | 4 | 180 ± 13 | 126 ± 11 | 1.47 ± 0.15** | |
| Retroperitoneal WAT | +/+ | 4 | 84 ± 28 | 41 ± 4 | 1.92 ± 0.52 |
| −/− | 4 | 71 ± 8 | 44 ± 2 | 1.61 ± 0.11 | |
| Femoral WAT | +/+ | 4 | 256 ± 47 | 358 ± 38 | 0.70 ± 0.06 |
| −/− | 4 | 362 ± 69 | 382 ± 32 | 1.00 ± 0.26 |
Tissue DNA content was measured fluorimetrically. Values are expressed as mean ± SE. *, P < 0.001; **, P < 0.05 versus HSL+/+ by Student's t test.
Figure 3.
Enlarged lipid droplets in adipocytes from BAT (a) and epididymal fat (WAT) (b) in wild-type and HSL−/− mice. (×50.) Distribution of cytoplasmic area is shown in the graphs: ●, wild-type; ○, HSL−/−. Median cytoplasmic area was increased by about 5-fold in BAT and by 2-fold in WAT.
Body weight was not significantly different, at least until 24 weeks of age, between wild-type and HSL−/− mice (36.1 ± 2.2 vs. 35.7 ± 1.2 g, P > 0.05, n = 13 in males; 29.3 ± 1.0 vs. 26.7 ± 0.8 g, P > 0.05, n = 14 in females). Overall VO2 (1.09 ± 0.05 vs. 1.23 ± 0.07 ml/min per mouse; P > 0.05, n = 6) and RQ (1.20 ± 0.02 vs. 1.12 ± 0.03; P > 0.05, n = 6) were not different between the wild-type and HSL−/− mice fed a normal chow. They were not different even when fasted or treated with BRL35135A, a β3-agonist (data not shown). Core body temperature changed from 38.5 ± 0.2°C to 38.1 ± 0.2°C in the wild-type mice (n = 6), and it changed from 38.3 ± 0.1°C to 37.6 ± 0.3°C in the HSL−/− mice (n = 6) after exposure to an atmospheric temperature of 4°C. Thus, the HSL−/− mice were as cold-sensitive as HSL+/+ mice.
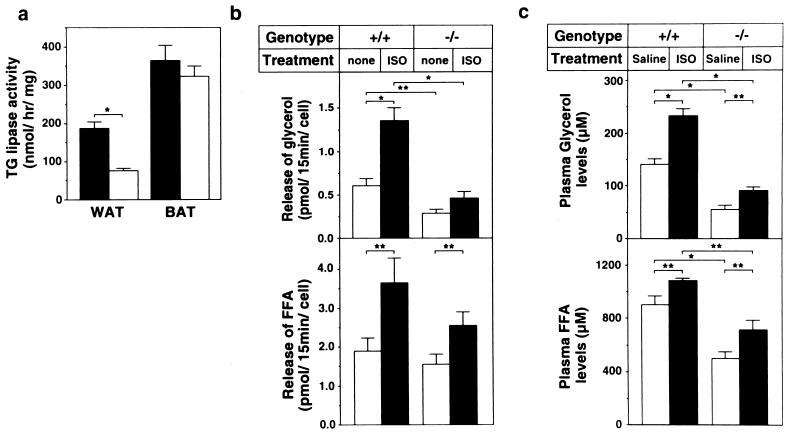
To determine how much triacylglycerol hydrolysis is accounted for by HSL in adipocytes, we measured triacylglycerol lipase activities in the presence of 1 M NaCl, which selectively inhibits LPL, and compared the lipolysis both in vitro and in vivo (Fig. 4). The HSL−/− WAT expressed significantly lower (40%), but still substantial, activities of triacylglycerol lipase as compared with the wild-type WAT (Fig. 4a). In BAT, there was no significant difference in the triacylglycerol lipase activities between wild-type and HSL−/− mice. In support of these results, adipocytes isolated from HSL−/− WAT released reduced, but still substantial, amounts of glycerol as well as FFA into the incubation media in vitro (Fig. 4b). Release of FFA, but not of glycerol, was stimulated by treatment with isoproterenol in the HSL−/− adipocytes. The isoproterenol-stimulated increment in the FFA concentration in HSL−/− WAT was smaller than that in wild-type WAT. In vivo, HSL−/− mice had lower plasma glycerol and FFA levels than wild-type mice (Fig. 4c). Although the increase in plasma glycerol concentration after the injection of isoproterenol was very small in HSL−/− mice as compared with wild-type mice, the increase in plasma FFA concentration was at least as great as that in the wild-type mice.
Figure 4.
The redundancy of triacylglycerol lipase. (a) Triacylglycerol lipase activities in WAT and BAT from the wild-type (solid bar) and HSL−/− mice (open bar) (n = 5). (b) In vitro lipolysis in adipocytes isolated from epididymal fat pad of wild-type (+/+; n = 12) and HSL−/− mice (−/−; n = 8). Adipocyte suspensions were treated with 100 μM isoproterenol (solid bar; ISO) or without it (open bar; none) for 15 min. Glycerol and FFA concentrations in the media were determined. (c) In vivo lipolysis. After a 12-h fast, 0.3 mg/kg of isoproterenol (solid bar; ISO) or saline (open bar; Saline) was injected intraperitoneally into control (+/+; n = 7) and HSL−/− (−/−; n = 10) mice. After 15 min, blood was collected and plasma concentrations of glycerol and FFA were determined. Data are expressed as mean ± SE. Mice used in these experiments were 12–16 weeks old. *, P < 0.001; **, P < 0.05 by Student's t test.
Discussion
Extremely divergent functions have been assigned to HSL: (i) liberation of FFA by triacylglycerol lipase activity in adipocytes, (ii) breakdown of cholesterol ester to supply cholesterol for steroidogenesis in adrenals, ovaries, and possibly testes, and (iii) breakdown of cholesterol ester in cells such as macrophages to prevent foam cell formation in arterial walls. Furthermore, it is highly expressed in spermatids and spermatozoa as a larger testicular isoform (HSLtes). In the current study, we have generated mice lacking HSL and used these animals to test these various functions.
The most striking phenotype of HSL−/− mice is male sterility caused by oligospermia. Holst et al. (10, 11) have shown that HSL is localized in elongating spermatids and spermatozoa, but not in interstitial cells such as Leydig cells. Consistent with the absence of HSL from Leydig cells, HSL−/− mice have normal plasma levels of testosterone, follicle-stimulating hormone, and luteinizing hormone. Thus the oligospermia does not result from hypogonadism. Rather, it is conceivable that HSL is directly involved in spermatogenesis in a manner similar to that of other genes whose functions are crucial for male germ cell differentiation but are not needed for oogenesis (30). Because HSL catalyzes the hydrolysis of cholesterol ester and triacylglycerol, it is reasonable to speculate that cholesterol or fatty acids released by the action of HSL are required for spermatogenesis. In this concern, it is noteworthy that cholesterol contents of sperms are significantly altered during epididymal maturation (31) and that deficiency of essential fatty acids leads to extensive testicular degeneration (32). It is also possible that the male sterility arises from a disturbance of retinoid metabolism, since HSL mediates retinol ester hydrolysis (33). It is well known that vitamin A is essential for reproductive function. For example, vitamin A-deficient rats develop a wide variety of phenotypes including male sterility (34). In addition, male sterility is the major phenotype of retinoid receptor RARα (35) and RXRβ (36) knockout mice that survive perinatal lethality. Further studies are needed to clarify the mechanism by which HSL deficiency causes male sterility.
Durham and Grogan (37) reported that two types of cholesterol ester hydrolases are present in the testes: temperature-stable and temperature-labile (TLCEH). TLCEH seems to be distinct from HSL, because of its different biochemical properties (38) and its localization in Sertoli cells (37). However, HSL may be related to TLCEH, because both enzymes have the following characteristics in common. They are more active at low temperature (28, 37) and their activity increases with sexual maturity (8, 39). When measured at 37°C, NCEH activities were barely detectable in the testes of HSL−/− mice (Fig. 1d), further supporting the hypothesis that HSL is related to TLCEH.
Phenotypes in adipocytes, where HSL is highly expressed, were milder than expected. Even though NCEH activities were completely absent and the mean size of adipocytes was increased, the adipose tissue mass was increased only in BAT and not in WAT in HSL−/− mice. In agreement with these observations, HSL−/− mice were not obese, judged from the growth curves. Since uncoupling protein (UCP) carries out thermogenesis using fatty acids as substrates in BAT, HSL−/− mice were expected to be sensitive to cold. However, this was not the case. Furthermore, there was no difference in the overall VO2, RQ, and core body temperature between the wild-type and HSL−/− mice, irrespective of the feeding status. These results indicate that fatty acids supplied from HSL-mediated lipolysis are not the only substrates utilized for the thermogenesis in BAT. Similar enlargement of lipid vacuoles in BAT adipocytes has been reported for mice lacking norepinephine and epinephrine (40) and UCP1 knockout mice (26). However, these mice are reportedly cold sensitive. Therefore, adipocyte hypertrophy in BAT and cold sensitivity appear to be independent phenotypes.
Phenotypes in WAT are interesting. Although the mean size of adipocytes in WAT was increased, total tissue mass was not. These results suggest the heterogeneity of adipocytes in WAT; a certain cell population is sensitive to lipid accumulation and the other is not. Alternatively, triacylglycerol may accumulate beyond the limit that the adipocytes can tolerate, thereby leading to disruption of cell integrity—i.e., death—in a population of adipocytes of HSL−/− mice.
It is noteworthy that WAT from HSL−/− mice retained substantial activities that hydrolyzed triacylglycerol in the presence of 1 M NaCl, an inhibitor of LPL (Fig. 4a). In agreement with these results, adipocytes isolated from HSL−/− epididymal fat released significant amounts of glycerol and FFA into the incubation media (Fig. 4b). This residual lipase activity appeared slightly responsive to isoproterenol. This is also consistent with the in vivo lipolysis experiments demonstrating that plasma FFA and glycerol were increased in response to isoproterenol treatment (Fig. 4c). Together, these results strongly indicate that HSL is not the only molecule that hydrolyzes triacylglycerol in adipocytes. This may be one of the reasons why HSL−/− mice were neither obese nor cold sensitive.
BAT expressed significantly lower activities of NCEH than WAT, and these activities were completely eliminated by the HSL disruption (Fig. 1d). On the other hand, BAT contained higher activities of triacylglycerol lipase than did WAT, but these activities were not affected by the HSL disruption (Fig. 4a). Considering these observations together, we speculate that the hydrolysis of triacylglycerol in BAT is primarily mediated by an enzyme that is distinct from HSL. This is also very consistent with the lack of cold sensitivity in HSL−/− mice.
Cholesterol-ester-laden foam cells are a hallmark of atherosclerosis. Cholesterol is in a dynamic equilibrium between free and esterified forms. The esterification and hydrolysis are mediated by acyl-CoA:cholesterol acyltransferase (ACAT) and NCEH, respectively. Therefore, it is believed that NCEH plays an important role in atherogenesis. Interestingly, NCEH activities expressed in arterial wall as well as in macrophage-like cell lines are hormone sensitive and are activated by protein kinase A (13, 14, 41, 42). Furthermore, Small et al. (16) have shown that the NCEH activities are completely inhibited by an anti-HSL antibody in the mouse macrophage cell line WEHI. Together with the fact that HSL is expressed in mouse peritoneal macrophages and P388D1 and J774 macrophage cell lines (17), it has been widely accepted that HSL mediates NCEH activity in macrophages. In contrast to this belief, our current results strongly indicate that the NCEH activity is accounted for by a molecule(s) distinct from HSL at least in mouse peritoneal macrophages (Fig. 1d). In this respect, it is noteworthy that human monocyte-derived macrophages may (43) or may not (19) express HSL, and their NCEH activity is not hormone sensitive (19).
In conclusion, we demonstrate that HSL is essential for spermatogenesis but is dispensable for lipolysis in adipocytes. These results also help to shed light on the debate about whether NCEH activity in macrophages is HSL.
Acknowledgments
We thank Y. Tsutsumi for determination of RQ, and K. Saito, M. Kusubae, and E. Herai for maintenance of animals. We also thank T. Kitamine, T. Gotoda, Y. Iizuka, Z. Chen, K. Ohashi, K. Harada, S. Perrey, Y. Tamura, and H. Shimano for helpful discussions. This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture, by the Promotion of Fundamental Studies in Health Science of The Organization for Pharmaceutical Safety and Research, by Health Sciences Research Grants (Research on Human Genome and Gene Therapy) from the Ministry of Health and Welfare, by the Research Service of the Department of Veterans Affairs, and by Grants DK 46942 and 49705 from the National Institutes of Health.
Abbreviations
- HSL
hormone-sensitive lipase
- LPL
lipoprotein lipase
- FFA
free fatty acid
- NCEH
neutral cholesterol ester hydrolase
- BAT
brown adipose tissue
- WAT
white adipose tissue
Footnotes
See commentary on page 535.
References
- 1.Strålfors P, Olsson H, Belfrage P. In: The Enzymes. Boyer P D, Krebs E G, editors. London: Academic; 1987. pp. 147–177. [Google Scholar]
- 2.Yeaman S J. Biochim Biophys Acta. 1990;1052:128–132. doi: 10.1016/0167-4889(90)90067-n. [DOI] [PubMed] [Google Scholar]
- 3.Holm C, Kirchgessner T G, Svenson K L, Fredrikson G, Nilsson S, Miller C G, Shively J E, Heinzmann C, Sparkes R S, Mohandas T, et al. Science. 1988;241:1503–1506. doi: 10.1126/science.3420405. [DOI] [PubMed] [Google Scholar]
- 4.Fredrikson G, Strålfors P, Nilsson N, Belfrage P. J Biol Chem. 1981;256:6311–6320. [PubMed] [Google Scholar]
- 5.Eriksson H, Ridderstrale M, Degerman E, Ekholm D, Smith C J, Manganiello V C, Belfrage P, Tornqvist H. Biochim Biophys Acta. 1996;1266:101–107. doi: 10.1016/0167-4889(94)00237-9. [DOI] [PubMed] [Google Scholar]
- 6.Susulic V S, Frederich R C, Lawitts J, Tozzo E, Kahn B B, Harper M-E, Himms-Hagen J, Flier J S, Lowell B B. J Biol Chem. 1995;270:29483–29492. doi: 10.1074/jbc.270.49.29483. [DOI] [PubMed] [Google Scholar]
- 7.Cummings D E, Brandon E P, Planas J V, Motamed K, Idzerda R L, McKnight G S. Nature (London) 1996;382:622–626. doi: 10.1038/382622a0. [DOI] [PubMed] [Google Scholar]
- 8.Kraemer F B, Tavanger K, Hoffman A R. J Lipid Res. 1991;32:1303–1310. [PubMed] [Google Scholar]
- 9.Hui D Y. Biochim Biophys Acta. 1996;1303:1–14. doi: 10.1016/0005-2760(96)00085-9. [DOI] [PubMed] [Google Scholar]
- 10.Holst L S, Hoffmann A M, Mulder H, Sundler F, Holm C, Bergh A, Fredrikson G. FEBS Lett. 1994;355:125–130. doi: 10.1016/0014-5793(94)01185-0. [DOI] [PubMed] [Google Scholar]
- 11.Holst L S, Langin D, Mulder H, Laurell H, Grober J, Bergh A, Mohrenweiser H W, Edgren G, Holm C. Genomics. 1996;35:441–447. doi: 10.1006/geno.1996.0383. [DOI] [PubMed] [Google Scholar]
- 12.Kraemer F B, Patel S, Saedi M S, Sztalryd C. J Lipid Res. 1993;34:663–671. [PubMed] [Google Scholar]
- 13.Hajjar D P, Minick C R, Fowler S. J Biol Chem. 1983;258:192–198. [PubMed] [Google Scholar]
- 14.Khoo J C, Mahoney E M, Steinberg D. J Biol Chem. 1981;256:12659–12661. [PubMed] [Google Scholar]
- 15.Escary J-L, Choy H A, Reue K, Schotz M C. Arterioscler Thromb Vasc Biol. 1998;18:991–998. doi: 10.1161/01.atv.18.6.991. [DOI] [PubMed] [Google Scholar]
- 16.Small C A, Goodacre J A, Yeaman S J. FEBS Lett. 1989;247:205–208. doi: 10.1016/0014-5793(89)81335-3. [DOI] [PubMed] [Google Scholar]
- 17.Small C A, Rogers M P, Goodacre J A, Yeaman S J. FEBS Lett. 1991;279:323–326. doi: 10.1016/0014-5793(91)80179-7. [DOI] [PubMed] [Google Scholar]
- 18.Khoo J C, Reue K, Steinberg D, Schotz M C. J Lipid Res. 1993;34:1969–1974. [PubMed] [Google Scholar]
- 19.Contreras J A, Lasunción M A. Arterioscler Thromb. 1994;14:443–452. doi: 10.1161/01.atv.14.3.443. [DOI] [PubMed] [Google Scholar]
- 20.Escary J-L, Choy H A, Reue K, Wang X-P, Castellani L W, Glass C K, Lusis A J, Schotz M C. J Lipid Res. 1999;40:397–404. [PubMed] [Google Scholar]
- 21.Ishibashi S, Brown M S, Goldstein J L, Gerard R D, Hammer R E, Herz J. J Clin Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradley D C, Kaslow H R. Anal Biochem. 1989;180:11–16. doi: 10.1016/0003-2697(89)90081-x. [DOI] [PubMed] [Google Scholar]
- 23.Tsutsumi O, Kurachi H, Oka T. Science. 1986;233:975–977. doi: 10.1126/science.3090686. [DOI] [PubMed] [Google Scholar]
- 24.Folch J, Lees M, Sloane-Stanley G H. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 25.Shimada M, Ishibashi S, Yamamoto K, Kawamura M, Watanabe Y, Gotoda T, Harada K, Inaba T, Ohsuga J, Yazaki Y, Yamada N. Biochem Biophys Res Commun. 1995;211:761–766. doi: 10.1006/bbrc.1995.1878. [DOI] [PubMed] [Google Scholar]
- 26.Enerbäck S, Jacobsson A, Simpson E M, Guerra C, Yamashita H, Harper M-E, Kozak L P. Nature (London) 1997;387:90–94. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- 27.Rodbell M. J Biol Chem. 1964;239:375–380. [PubMed] [Google Scholar]
- 28.Langin D, Laurell H, Holst L S, Belfrage P, Holm C. Proc Natl Acad Sci USA. 1993;90:4897–4901. doi: 10.1073/pnas.90.11.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Contreras J A, Karlsson M, Østerlund T, Laurell H, Svensson A, Holm C. J Biol Chem. 1996;271:31426–31430. doi: 10.1074/jbc.271.49.31426. [DOI] [PubMed] [Google Scholar]
- 30.Sassone-Corsi P. Cell. 1997;88:163–166. doi: 10.1016/s0092-8674(00)81834-6. [DOI] [PubMed] [Google Scholar]
- 31.Rana A P, Majumder G C, Misra S, Ghosh A. Biochim Biophys Acta. 1991;1061:185–196. doi: 10.1016/0005-2736(91)90284-f. [DOI] [PubMed] [Google Scholar]
- 32.MacDonald M L, Rogers Q R, Morris J G, Cupps P T. J Nutr. 1984;114:719–726. doi: 10.1093/jn/114.4.719. [DOI] [PubMed] [Google Scholar]
- 33.Wei S, Lai K, Patel S, Piantedosi R, Shen H, Colantuoni V, Kraemer F B, Blaner W S. J Biol Chem. 1997;272:14159–14165. doi: 10.1074/jbc.272.22.14159. [DOI] [PubMed] [Google Scholar]
- 34.Esklid W, Hansson V. In: Vitamin A in Health and Disease. Blomhoff R, editor. New York: Dekker; 1994. pp. 531–559. [Google Scholar]
- 35.Lufkin T, Lohnes D, Mark M, Dierich A, Gorry P, Gaub M P, LeMeur M, Chambon P. Proc Natl Acad Sci USA. 1993;90:7225–7229. doi: 10.1073/pnas.90.15.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kastner P, Mark M, Leid M, Gansmuller A, Chin W, Grondona J M, Décimo D, Krezel W, Dierich A, Chambon P. Genes Dev. 1996;10:80–92. doi: 10.1101/gad.10.1.80. [DOI] [PubMed] [Google Scholar]
- 37.Durham L A, III, Grogan W M. J Biol Chem. 1984;259:7433–7438. [PubMed] [Google Scholar]
- 38.Wee S F, Grogan W M. J Biol Chem. 1993;268:8158–8163. [PubMed] [Google Scholar]
- 39.Wee S F, Grogan W M. Lipids. 1989;24:824–828. doi: 10.1007/BF02544591. [DOI] [PubMed] [Google Scholar]
- 40.Thomas S A, Palmiter R D. Nature (London) 1997;387:94–97. doi: 10.1038/387094a0. [DOI] [PubMed] [Google Scholar]
- 41.Goldberg D I, Khoo J C. Biochim Biophys Acta. 1990;1042:132–137. doi: 10.1016/0005-2760(90)90067-8. [DOI] [PubMed] [Google Scholar]
- 42.Bernard D W, Rodriguez A, Rothblat G H, Glick J M. J Biol Chem. 1991;266:710–716. [PubMed] [Google Scholar]
- 43.Reue K, Choen R D, Schotz M C. Arterioscler Thromb Vasc Biol. 1997;17:3428–3432. doi: 10.1161/01.atv.17.12.3428. [DOI] [PubMed] [Google Scholar]