Abstract
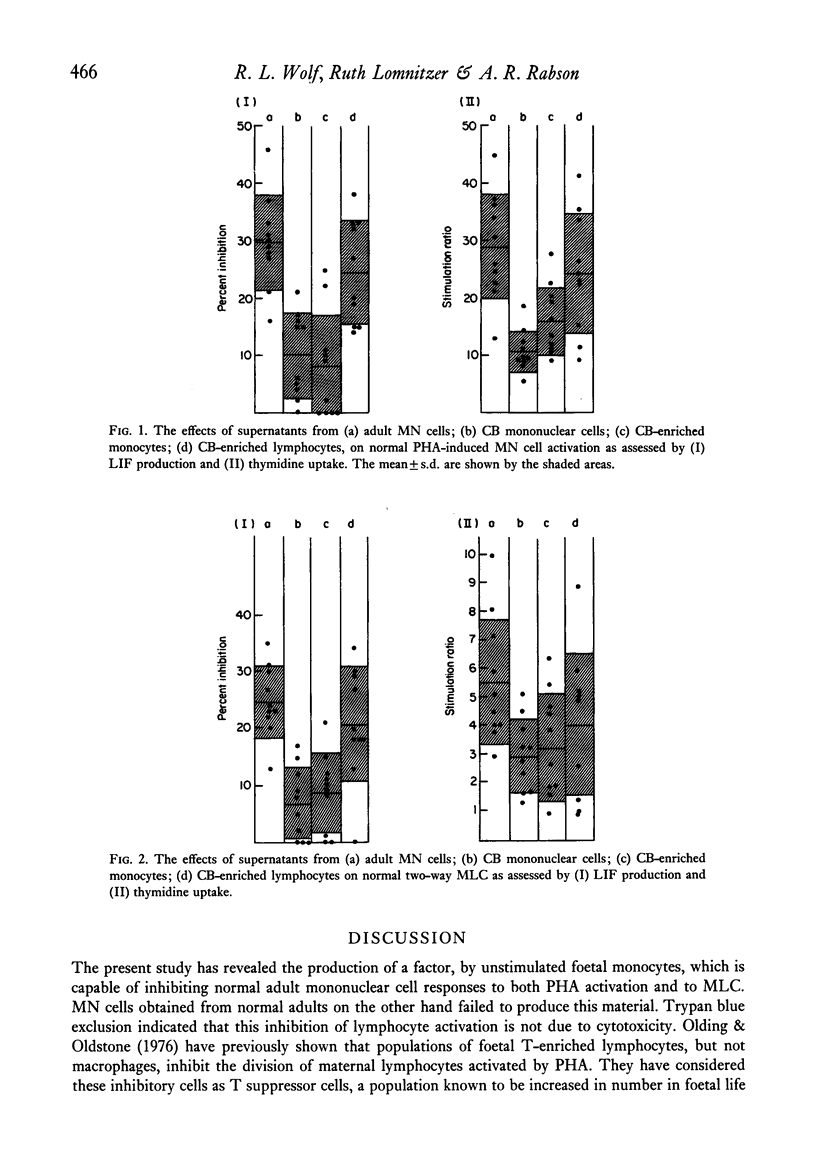
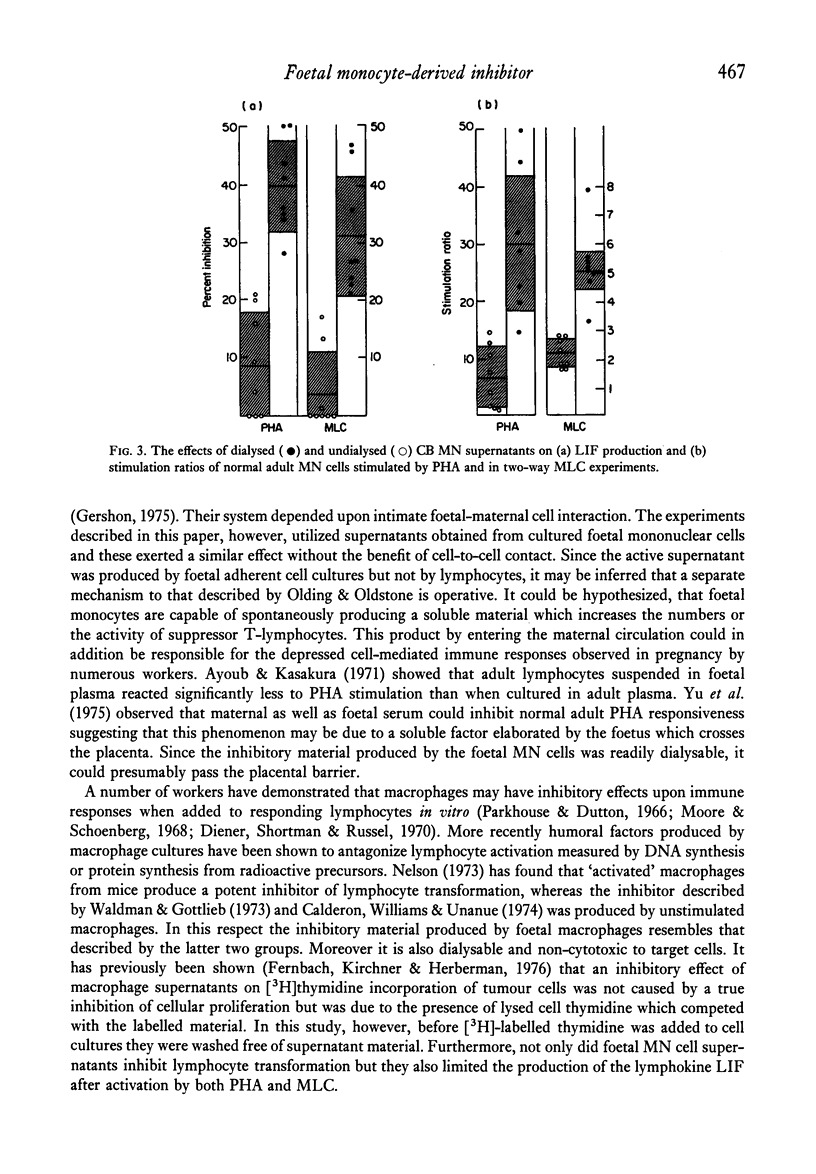
Supernatants obtained from 3-day cultures of cord blood monocytes inhibited normal lymphocyte activation by either PHA or in a two-way MLC. Both lymphocyte transformation and lymphokine production was significantly inhibited by these supernatants but not by those derived from adult mononuclear cell cultures. The inhibitory material produced by foetal monocytes was dialysable and was non-cytotoxic to target cells. It is postulated that this factor contributes to the depressed maternal cell-mediated immune response observed in pregnancy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayoub J., Kasakura S. In vitro response of foetal lymphocytes to PHA, and a factor plasma which suppresses the PHA response of adult lymphocytes. Clin Exp Immunol. 1971 Mar;8(3):427–434. [PMC free article] [PubMed] [Google Scholar]
- Diener E., Shortman K., Russell P. Induction of immunity and tolerance in vitro in the absence of phagocytic cells. Nature. 1970 Feb 21;225(5234):731–732. doi: 10.1038/225731a0. [DOI] [PubMed] [Google Scholar]
- Erb P., Feldmann M. Role of macrophages in in vitro induction of T-helper cells. Nature. 1975 Mar 27;254(5498):352–354. doi: 10.1038/254352a0. [DOI] [PubMed] [Google Scholar]
- Fernbach B. R., Kirchner H., Herberman R. B. Inhibition of the mixed lymphocyte culture by peritoneal exudate cells. Cell Immunol. 1976 Mar 15;22(2):399–403. doi: 10.1016/0008-8749(76)90043-5. [DOI] [PubMed] [Google Scholar]
- Finn R., St Hill C. A., Govan A. J., Ralfs I. G., Gurney F. J., Denye V. Immunological responses in pregnancy and survival of fetal homograft. Br Med J. 1972 Jul 15;3(5819):150–152. doi: 10.1136/bmj.3.5819.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. R., Kearns D. R. Investigation of the structure of yeast tRNAphe by nuclear magnetic resonance: paramagnetic rare earth ion probes of structure. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4237–4240. doi: 10.1073/pnas.71.10.4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E., Curzen P. The immunological reactivity of maternal lymphocytes in pregnancy. J Obstet Gynaecol Br Commonw. 1973 Jul;80(7):608–610. doi: 10.1111/j.1471-0528.1973.tb16034.x. [DOI] [PubMed] [Google Scholar]
- Kasakura S. A factor in maternal plasma during pregnancy that suppresses the reactivity of mixed leukocyte cultures. J Immunol. 1971 Nov;107(5):1296–1301. [PubMed] [Google Scholar]
- Lawler S. D., Ukaejiofo E. O., Reeves B. R. Interaction of maternal and neonatal cells in mixed-lymphocyte cultures. Lancet. 1975 Dec 13;2(7946):1185–1187. doi: 10.1016/s0140-6736(75)92663-x. [DOI] [PubMed] [Google Scholar]
- Moore R. D., Schoenberg M. D. Restimulation of antibody synthesis by antigen in cultures of lymphocytes. Nature. 1968 Jul 20;219(5151):297–298. doi: 10.1038/219297a0. [DOI] [PubMed] [Google Scholar]
- Olding L. B., Oldstone B. A. Thymus-derived peripheral lymphocytes from human newborns inhibit division of their mothers' lymphocytes. J Immunol. 1976 Mar;116(3):682–686. [PubMed] [Google Scholar]
- Olding L. B., Oldstone M. B. Lymphocytes from human newborns abrogate mitosis of their mother's lymphocytes. Nature. 1974 May 10;249(453):161–162. doi: 10.1038/249161a0. [DOI] [PubMed] [Google Scholar]
- Parkhouse R. M., Dutton R. W. Inhibition of spleen cell DNA synthesis by autologous macrophages. J Immunol. 1966 Nov;97(5):663–669. [PubMed] [Google Scholar]
- Purtilo D. T., Hallgren H. M., Yunis E. J. Depressed maternal lymphocyte response to phytohaemagglutinin in human pregnancy. Lancet. 1972 Apr 8;1(7754):769–771. doi: 10.1016/s0140-6736(72)90522-3. [DOI] [PubMed] [Google Scholar]
- Rabson A. R., Bey M. C., Kerrich J. E., Koornhof H. J. The blocking by autologous serum of maternal cell-mediated immune reactions to placental antigen. S Afr Med J. 1976 Feb 14;50(7):201–205. [PubMed] [Google Scholar]
- Rocklin R. E. Products of activated lymphocytes: leukocyte inhibitory factor (LIF) distinct from migration inhibitory factor (MIF). J Immunol. 1974 Apr;112(4):1461–1466. [PubMed] [Google Scholar]
- Stimson W. H. Transplantation--nature's success. Lancet. 1972 Mar 25;1(7752):684–684. doi: 10.1016/s0140-6736(72)90484-9. [DOI] [PubMed] [Google Scholar]
- Than G. N., Csaba I. F., Szabó D. G. Letter: An antigen associated with pregnancy. Lancet. 1974 Dec 28;2(7896):1578–1579. doi: 10.1016/s0140-6736(74)90333-x. [DOI] [PubMed] [Google Scholar]
- Waldman S. R., Gottlieb A. A. Macrophage regulation of DNA synthesis in lymphoid cells: effects of a soluble factor from macrophages. Cell Immunol. 1973 Oct;9(1):142–156. doi: 10.1016/0008-8749(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Yoshinaga M., Yoshinaga A., Waksman B. H. Regulation of lymphocyte responses in vitro. I. Regulatory effect of macrophages and thymus-dependent (T) cells on the response of thymus-independent (B) lymphocytes to endotoxin. J Exp Med. 1972 Oct 1;136(4):956–961. doi: 10.1084/jem.136.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu V. Y., Waller C. A., Maclennan I. C., Baum J. D. Lymphocyte reactivity in pregnant women and newborn infants. Br Med J. 1975 Feb 22;1(5955):428–432. doi: 10.1136/bmj.1.5955.428. [DOI] [PMC free article] [PubMed] [Google Scholar]


