Abstract
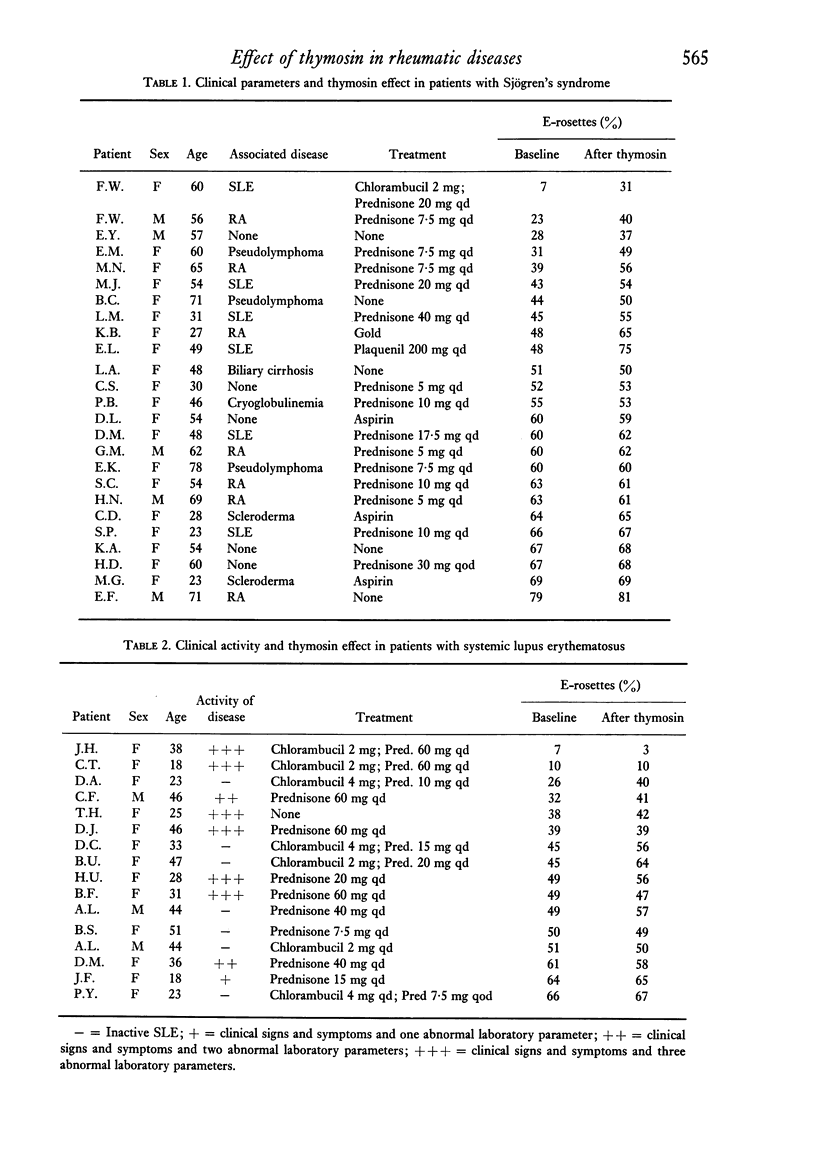
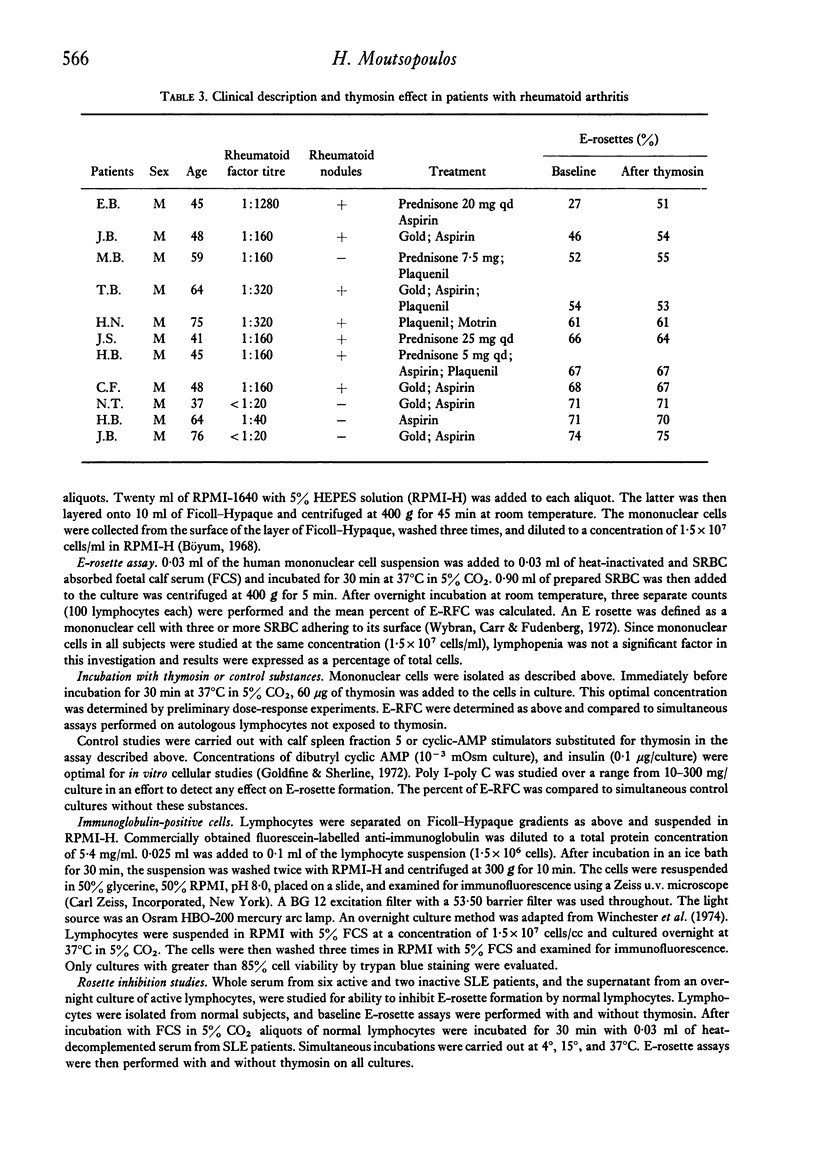
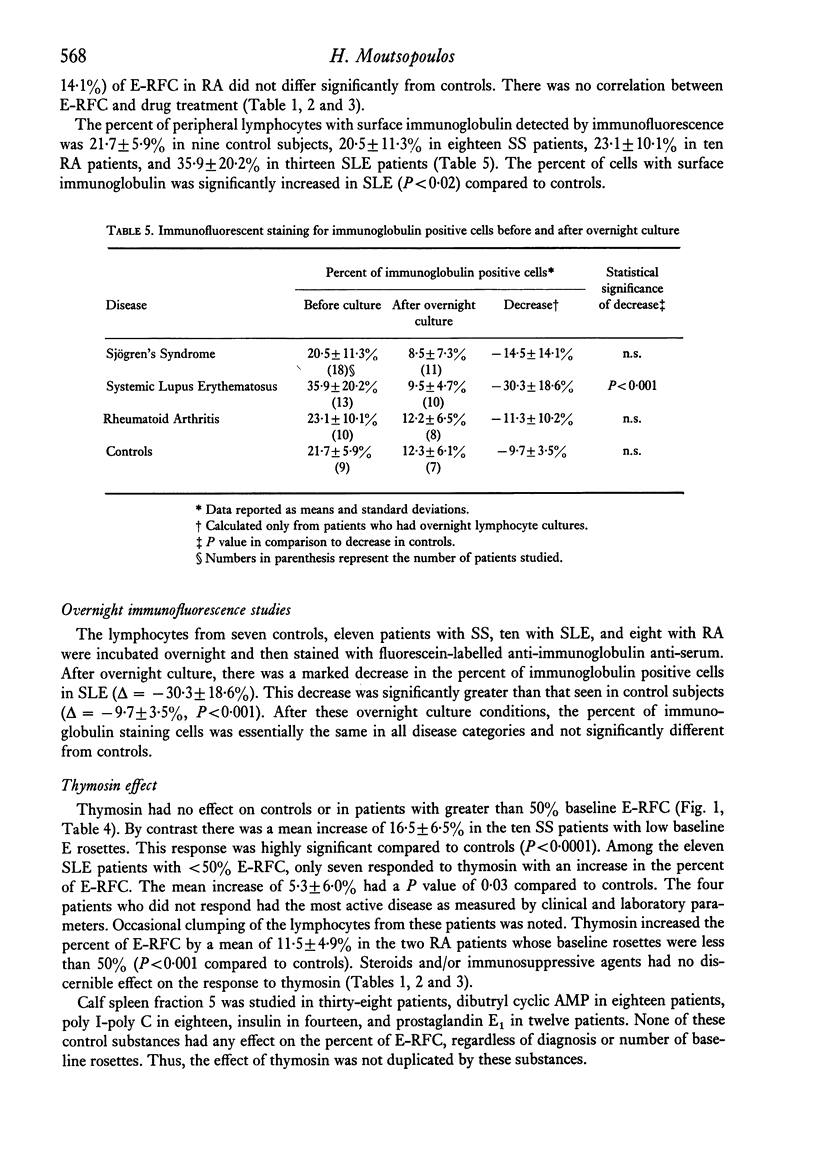
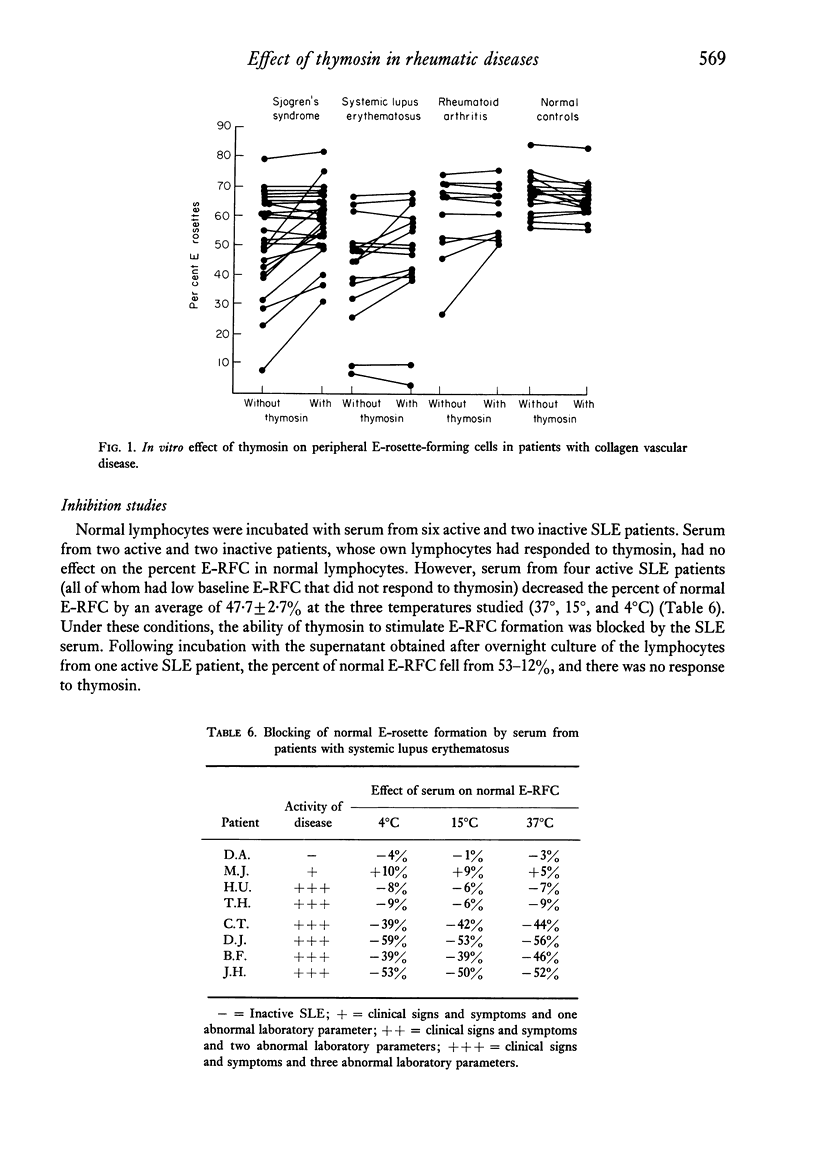
The in vitro effect of calf thymosin fraction 5 on T-rosette forming cells (E-RFC) was studied in Sjögren's syndrome (SS), rheumatoid arthritis (RA), and systemic lupus erythematosus (SLE). The baseline percent E-RFC in sixteen normal controls was67-2 +/- 6-9. E-RFC was significantly decreased in SLE (42-6 +/- 17-0, P less than 0-0001) and SS (51-8 +/- 16-9, P less than 0-002) but not in RA (59-7 +/- 14-1). Ten of twenty-five SS patients and two of eleven RA patients had less than 50% E-RFC, and all showed a significant increase after incubation with thymosin (+ 16-5 +/- 6-5%, P less than 0-0001, and + 11 +/- 4-9%, P less than 0-001, respectively). Eleven of sixteen SLE patients had less than 50% E-RFC. Their response to thymosin was less dramatic but still statistically significant (+ 5-3 +/- 6-0%, P = 0-03). There was no response to thymosin in control subjects or in patients with baseline E-RFC greater than 50%. No increase in E-RFC was seen after incubation with calf spleen fraction 5 or known stimulators of cyclic-AMP. Sera from four active SLE patients, as well as the supernatant obtained from overnight culture of the lymphocytes from one SLE patients, were able to block T-rosette formation by normal lymphocytes, even after exposure to thymosin. Two 'blocking' sera were fractionated by sucrose density gradient ultracentrifucation. In one, the blocking capacity was found to reside in the 19S region containing IgM. In the second, the blocking capacity was in the 7S region containing IgG. Four 'blocking' lupus sera were depleted of IgG or IgM by immunoabsorption with goat anti-human IgG or goat anti-human IgM sepharose 4B. The blocking ability in three sera was partially decreased by depletion of either IgG or IgM, and in a fourth, only by removing IgG. The percent of lymphocytes staining with fluorescein labelled goat anti-human immunoglobulin antisera was increased in SLE patients (35-9 +/- 20-2 vs 21-7 +/- 5-9 in controls, P = 0-02). After overnight culture, the percent of staining cells decreased to normal values. These results suggest that thymosin can stimulate the differentiation of T-lymphocytes in patients with SS, SLE, and RA when the baseline E-RFC is decreased. Furthermore, the decreased percent E-RFC in SLE is probably due to cell-bound anti-lymphocyte antibodies that block sheep erythrocyte receptors on the T-cell and, possibly, thymosin receptors on undifferentiated lymphocytes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carnegie P. R., Mackay I. R. Vulnerability of cell-surface receptors to autoimmune reactions. Lancet. 1975 Oct 11;2(7937):684–687. doi: 10.1016/s0140-6736(75)90779-5. [DOI] [PubMed] [Google Scholar]
- Daniels T. E., Silverman S., Jr, Michalski J. P., Greenspan J. S., Sylvester R. A., Talal N. The oral component of Sjögren's syndrome. Oral Surg Oral Med Oral Pathol. 1975 Jun;39(6):875–885. doi: 10.1016/0030-4220(75)90108-5. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Kahn C. R., Roth J., Bar R. S. Antibodies that impair insulin receptor binding in an unusual diabetic syndrome with severe insulin resistance. Science. 1975 Oct 3;190(4209):63–65. doi: 10.1126/science.170678. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Sherline P. Insulin action in isolated rat thymocytes. II. Independence of insulin and cyclic adenosine monophosphate. J Biol Chem. 1972 Nov 10;247(21):6927–6931. [PubMed] [Google Scholar]
- Goldstein A. L., Guha A., Zatz M. M., Hardy M. A., White A. Purification and biological activity of thymosin, a hormone of the thymus gland. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1800–1803. doi: 10.1073/pnas.69.7.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. L., Slater F. D., White A. Preparation, assay, and partial purification of a thymic lymphocytopoietic factor (thymosin). Proc Natl Acad Sci U S A. 1966 Sep;56(3):1010–1017. doi: 10.1073/pnas.56.3.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro K., Boyse E. A. In-vitro demonstration of thymic hormone in the mouse by conversion of precursor cells into lymphocytes. Lancet. 1973 Apr 7;1(7806):740–743. doi: 10.1016/s0140-6736(73)92127-2. [DOI] [PubMed] [Google Scholar]
- Kook A. I., Trainin N. Hormone-like activity of a thymus humoral factor on the induction of immune competence in lymphoid cells. J Exp Med. 1974 Jan 1;139(1):193–207. doi: 10.1084/jem.139.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx J. L. Thymic hormones: inducers of T cell maturation. Science. 1975 Mar 28;187(4182):1183–1217. doi: 10.1126/science.187.4182.1183. [DOI] [PubMed] [Google Scholar]
- Messner R. P., Lindström F. D., Williams R. C., Jr Peripheral blood lymphocyte cell surface markers during the course of systemic lupus erythematosus. J Clin Invest. 1973 Dec;52(12):3046–3056. doi: 10.1172/JCI107503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSOBA D., MILLER J. F. THE LYMPHOD TISSUES AND IMMUNE RESPONSES OF NEONATALLY THYMECTOMIZED MICE BEARING THYMUS TISSUE IN MILLIPORE DIFFUSION CHAMBERS. J Exp Med. 1964 Jan 1;119:177–194. doi: 10.1084/jem.119.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick J., Lindstrom J. Autoimmune response to acetylcholine receptor. Science. 1973 May 25;180(4088):871–872. doi: 10.1126/science.180.4088.871. [DOI] [PubMed] [Google Scholar]
- Pillarisetty R. J., Becker M. J., Palmer D. W., Talal N. Antibodies binding polyriboadenylic acid in systemic lupus erythematosus. Immunochemical characterization and isolation by affinity chromatography. Clin Exp Immunol. 1975 Dec;22(3):419–425. [PMC free article] [PubMed] [Google Scholar]
- ROPES M. W., BENNETT G. A., COBB S., JACOX R., JESSAR R. A. 1958 Revision of diagnostic criteria for rheumatoid arthritis. Bull Rheum Dis. 1958 Dec;9(4):175–176. [PubMed] [Google Scholar]
- Scheid M. P., Hoffmann M. K., Komuro K., Hämmerling U., Abbott J., Boyse E. A., Cohen G. H., Hooper J. A., Schulof R. S., Goldstein A. L. Differentiation of T cells induced by preparations from thymus and by nonthymic agents. J Exp Med. 1973 Oct 1;138(4):1027–1032. doi: 10.1084/jem.138.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinberg M. A., Cathcart E. S. B cell and T cell lymphopenia in systemic lupus erythematosus. Cell Immunol. 1974 May;12(2):309–314. doi: 10.1016/0008-8749(74)90083-5. [DOI] [PubMed] [Google Scholar]
- Scheinberg M. A., Cathcart E. S., Goldstein A. L. Thymosin-induced reduction of "null cells" in peripheral-blood lymphocytes of patients with systemic lupus erythematosus. Lancet. 1975 Feb 22;1(7904):424–426. doi: 10.1016/s0140-6736(75)91491-9. [DOI] [PubMed] [Google Scholar]
- Steele R. W., Limas C., Thurman G. B., Schuelein M., Bauer H., Bellanti J. A. Familial thymic aplasia. Attempted reconstitution with fetal thymus in a Millipore diffusion chamber. N Engl J Med. 1972 Oct 19;287(16):787–791. doi: 10.1056/NEJM197210192871602. [DOI] [PubMed] [Google Scholar]
- Talal N., Dauphinee M., Pillarisetty R., Goldblum R. Effect of thymosin on thymocyte proliferation and autoimmunity in NZB mice. Ann N Y Acad Sci. 1975 Feb 28;249:438–450. doi: 10.1111/j.1749-6632.1975.tb29092.x. [DOI] [PubMed] [Google Scholar]
- Talal N., Sylvester R. A., Daniels T. E., Greenspan J. S., Williams R. C., Jr T and B lymphocytes in peripheral blood and tissue lesions in Sjögren's syndrome. J Clin Invest. 1974 Jan;53(1):180–189. doi: 10.1172/JCI107536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainin N. Thymic hormones and the immune response. Physiol Rev. 1974 Apr;54(2):272–315. doi: 10.1152/physrev.1974.54.2.272. [DOI] [PubMed] [Google Scholar]
- Wara D. W., Goldstein A. L., Doyle N. E., Ammann A. J. Thymosin activity in patients with cellular immunodeficiency. N Engl J Med. 1975 Jan 9;292(2):70–74. doi: 10.1056/NEJM197501092920204. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Jr, DeBoard J. R., Mellbye O. J., Messner R. P., Lindström F. D. Studies of T- and B-lymphocytes in patients with connective tissue diseases. J Clin Invest. 1973 Feb;52(2):283–295. doi: 10.1172/JCI107184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchester R. J., Winfield J. B., Siegal F., Wernet P., Bentwich Z., Kunkel H. G. Analyses of lymphocytes from patients with rheumatoid arthritis and systemic lupus erythematosus. Occurrence of interfering cold-reactive antilymphocyte antibodies. J Clin Invest. 1974 Nov;54(5):1082–1092. doi: 10.1172/JCI107852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wybran J., Carr M. C., Fudenberg H. H. The human rosette-forming cell as a marker of a population of thymus-derived cells. J Clin Invest. 1972 Oct;51(10):2537–2543. doi: 10.1172/JCI107069. [DOI] [PMC free article] [PubMed] [Google Scholar]


