Abstract
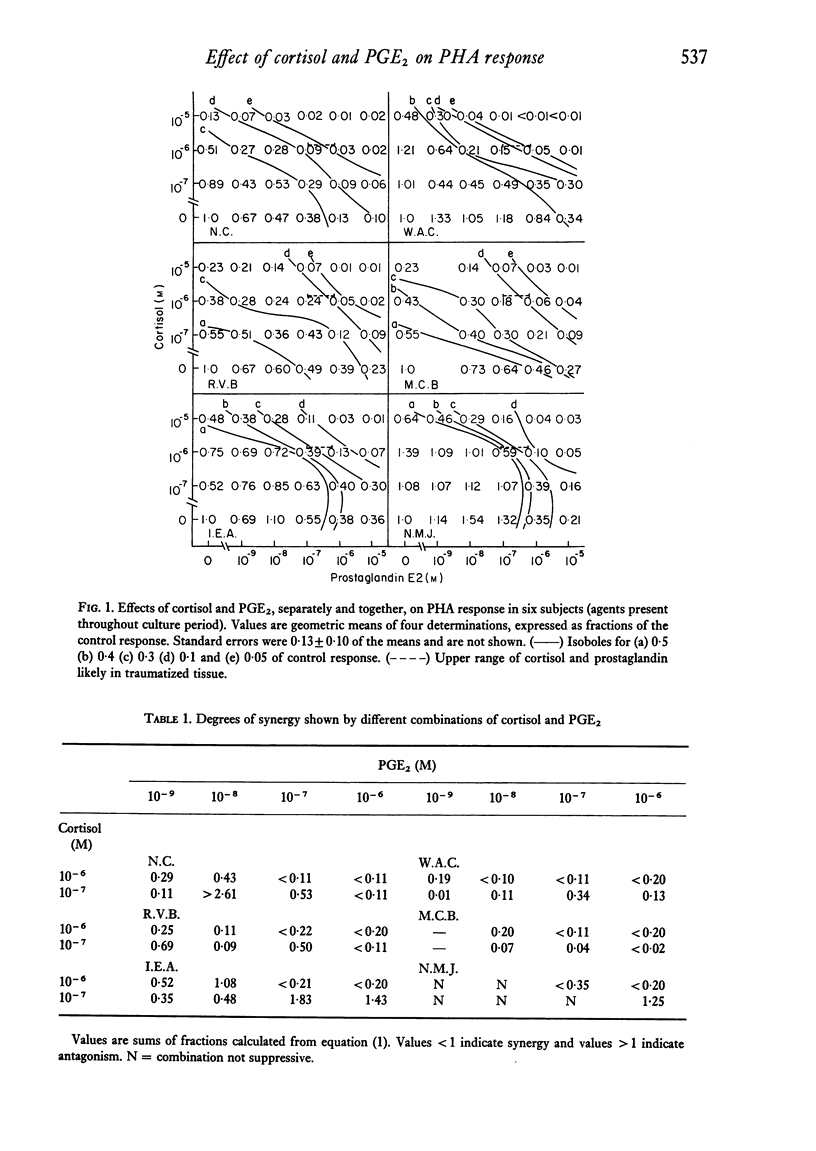
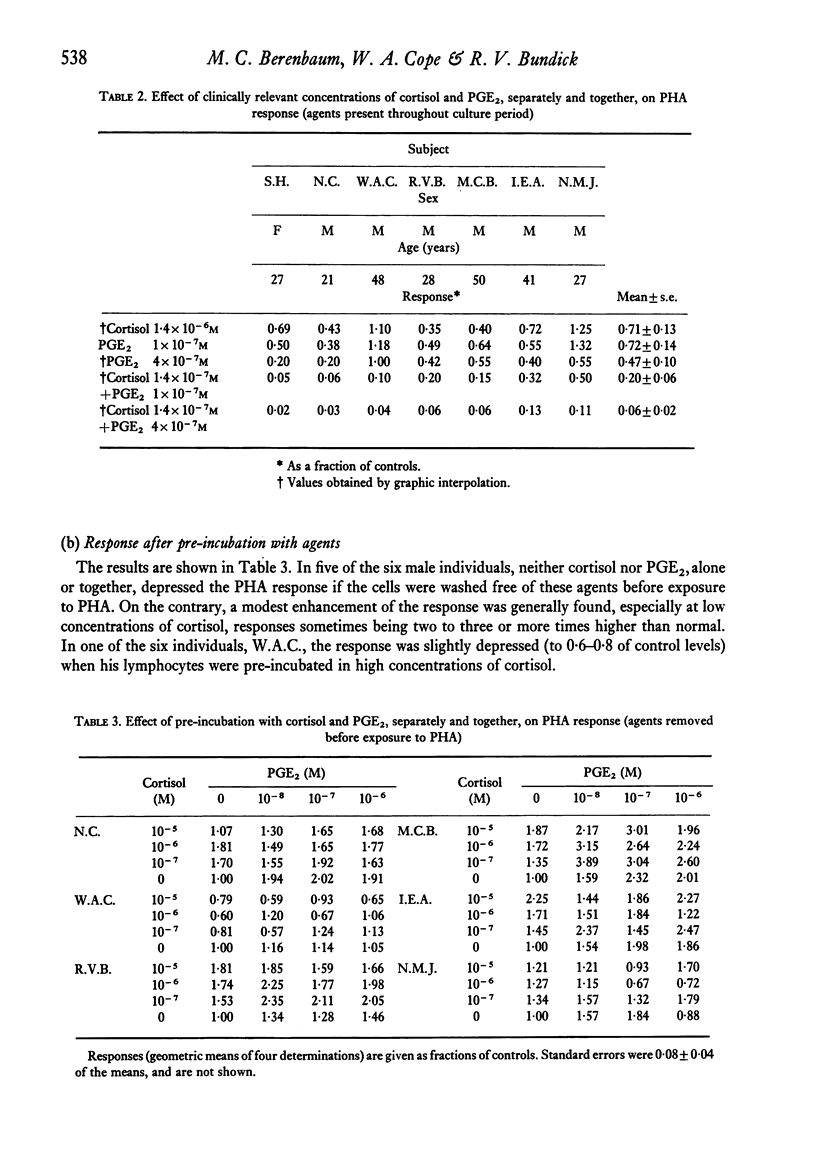
Surgical and thermal trauma in man are followed by depressed immunological responses in vivo and reduced lymphocyte reactivity in vitro. The possibility that these are related to trauma-induced rises in tissue levels of cortisol and prostaglandins was examined by studying the effect of a wide range of concentrations of cortisol and prostaglandin E2 (PGE2), separately and together on the phytohaemagglutinin (PHA) response of human peripheral blood lymphocytes. These effects were plotted on two-dimensional dose:effect graphs; the shapes of the curves connecting combinations of equal effect (isoboles) showed that these agents acted with marked synergy in suppressing the response, provided they were present while the response was taking place. Synergy was also shown by using a simple equation relating the concentrations of the agents producing a given effect when used in combination to the concentrations needed to produce the same effect when used separately. Cortisol at concentrations reached in the peripheral blood after trauma in man (1-4 X 10(-6)M) and PGE2 at concentrations to be expected in traumatized tissues (up to 4 X 10(-7)M) each suppressed the response only slightly. The former reduced the response to 0-7 of controls and the latter 0-5 (means of seven subjects). When both were present together at these concentrations, the response was markedly depressed (mean 0-06, range 0-02--0-13 of controls). However, when lymphocytes were incubated at 37 degrees C with cortisol and PGE2 for 20 hr and then washed before exposure to PHA, the response was not inhibited, even by substantially higher concentrations than the above, and was usually moderately enhanced. Therefore, these in vitro experiments do not explain the depressed PHA response observed in peripheral blood lymphocytes after trauma. It is possible, however, that raised cortisol and prostaglandin levels depress the reactivity of lymphocytes while they remain in the traumatized region and its lymph drainage area.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. W., Moncrief J. A. Alterations of the immune response following severe thermal injury. Arch Surg. 1966 Jul;93(1):75–83. doi: 10.1001/archsurg.1966.01330010077011. [DOI] [PubMed] [Google Scholar]
- Arturson G., Hallén A., Hansson H. E., Jonsson C. E. Efflux of prostaglandin-like smooth-muscle-stimulating activity in the right lymphatic duct in man. Scand J Clin Lab Invest. 1974 Apr;33(2):131–137. [PubMed] [Google Scholar]
- Arturson G., Hamberg M., Jonsson C. E. Prostaglandins in human burn blister fluid. Acta Physiol Scand. 1973 Feb;87(2):270–276. doi: 10.1111/j.1748-1716.1973.tb05390.x. [DOI] [PubMed] [Google Scholar]
- Bancewicz J., Gray A. C., Lindop G. The immunosuppressive effect of surgery--a possible mechanism. Br J Surg. 1973 Apr;60(4):314–315. [PubMed] [Google Scholar]
- Bane J. W., McCaa R. E., McCaa C. S., Read V. H., Turney W. H., Turner M. D. The pattern of aldosterone and cortisone blood levels in thermal burn patients. J Trauma. 1974 Jul;14(7):605–611. doi: 10.1097/00005373-197407000-00008. [DOI] [PubMed] [Google Scholar]
- Bennett A., McDonald A. M., Simpson J. S., Stamford I. F. Breast cancer, prostaglandins, and bone metastases. Lancet. 1975 May 31;1(7918):1218–1220. doi: 10.1016/s0140-6736(75)92197-2. [DOI] [PubMed] [Google Scholar]
- Berenbaum M. C., Fluck P. A., Hurst N. P. Depression of lymphocyte responses after surgical trauma. Br J Exp Pathol. 1973 Dec;54(6):597–607. [PMC free article] [PubMed] [Google Scholar]
- Bergman S., Borgström S., Tärnvik A. Lymphocyte response to phytohaemagglutinin following cholecystectomy. Acta Pathol Microbiol Scand. 1969;75(3):363–366. [PubMed] [Google Scholar]
- Carter M. E., James V. H. Pituitary-adrenal response to surgical stress in patients receiving corticotrophin treatment. Lancet. 1970 Feb 14;1(7642):328–330. doi: 10.1016/s0140-6736(70)90704-x. [DOI] [PubMed] [Google Scholar]
- Casson P., Solowey A. C., Converse J. M., Rapaport F. T. Delayed hypersensitivity status of burned patients. Surg Forum. 1966;17:268–270. [PubMed] [Google Scholar]
- Cheigh J. S., Stenzel K. H., Riggio R. R., Katz E. B., Rubin A. L. Effects of intravenous methylprednisolone on mixed lymphocyte cultures in normal humans. Transplant Proc. 1975 Mar;7(1):31–35. [PubMed] [Google Scholar]
- Cochran A. J., Spilg W. G., Mackie R. M., Thomas C. E. Postoperative depression of tumour-directed cell-mediated immunity in patients with malignant disease. Br Med J. 1972 Oct 14;4(5832):67–70. [PMC free article] [PubMed] [Google Scholar]
- Eakins K. E., Whitelocke R. A., Bennett A., Martenet A. C. Prostaglandin-like activity in ocular inflammation. Br Med J. 1972 Aug 19;3(5824):452–453. doi: 10.1136/bmj.3.5824.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S., Dale D. C. Alternate-day prednisone therapy and human lymphocyte subpopulations. J Clin Invest. 1975 Jan;55(1):22–32. doi: 10.1172/JCI107914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S., Dale D. C. The effect of in vivo hydrocortisone on subpopulations of human lymphocytes. J Clin Invest. 1974 Jan;53(1):240–246. doi: 10.1172/JCI107544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira S. H., Vane J. R. Prostaglandins: their disappearance from and release into the circulation. Nature. 1967 Dec 2;216(5118):868–873. doi: 10.1038/216868a0. [DOI] [PubMed] [Google Scholar]
- Golub M., Zia P., Matsuno M., Horton R. Metabolism of prostaglandins A1 and E1 in man. J Clin Invest. 1975 Dec;56(6):1404–1410. doi: 10.1172/JCI108221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadden J. W., Hadden E. M., Middleton E., Jr Lymphocyte blast transformation. I. Demonstration of adrenergic receptors in human peripheral lymphocytes. Cell Immunol. 1970 Dec;1(6):583–595. doi: 10.1016/0008-8749(70)90024-9. [DOI] [PubMed] [Google Scholar]
- Jaffe B. M., Behrman H. R., Parker C. W. Radioimmunoassay measurement of prostaglandins E, A, and F in human plasma. J Clin Invest. 1973 Feb;52(2):398–405. doi: 10.1172/JCI107196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jättelä A., Alho A., Avikainen V., Karaharju E., Kataja J., Lahdensuu M., Lepistö P., Rokkanen P., Tervo T. Plasma catecholamines in severely injured patients: a prospective study on 45 patients with multiple injuries. Br J Surg. 1975 Mar;62(3):177–181. doi: 10.1002/bjs.1800620303. [DOI] [PubMed] [Google Scholar]
- LOEWE S. The problem of synergism and antagonism of combined drugs. Arzneimittelforschung. 1953 Jun;3(6):285–290. [PubMed] [Google Scholar]
- Leguit P., Jr, Meinesz A., Zeijlemaker W. P., Schellekens P. T., Eijsvoogel V. P. Immunological studies in burn patients. I. Lymphocyte transformation in vitro. Int Arch Allergy Appl Immunol. 1973;44(1):101–121. doi: 10.1159/000230921. [DOI] [PubMed] [Google Scholar]
- McCabe W. P., Rebuck J. W., Kelly A. P., Jr, Ditmars D. M., Jr Leukocytic response as a monitor of immunodepression in burn patients. Arch Surg. 1973 Feb;106(2):155–159. doi: 10.1001/archsurg.1973.01350140021008. [DOI] [PubMed] [Google Scholar]
- Mendelsohn J., Multer M. M., Boone R. F. Enhanced effects of prostaglandin E1 and dibutyryl cyclic AMP upon human lymphocytes in the presence of cortisol. J Clin Invest. 1973 Sep;52(9):2129–2137. doi: 10.1172/JCI107397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster A. M., Eurenius K., Katz R. M., Canales L., Foley F. D., Mortensen R. F. Cell-mediated immunity after thermal injury. Ann Surg. 1973 Feb;177(2):139–143. doi: 10.1097/00000658-197302000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plescia O. J., Smith A. H., Grinwich K. Subversion of immune system by tumor cells and role of prostaglandins. Proc Natl Acad Sci U S A. 1975 May;72(5):1848–1851. doi: 10.1073/pnas.72.5.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle P. R., Berenbaum M. C. Postoperative depression of the lymphocyte response to phytohaemagglutinin. Lancet. 1967 Apr 8;1(7493):746–748. doi: 10.1016/s0140-6736(67)91364-5. [DOI] [PubMed] [Google Scholar]
- Sakai H., Daniels J. C., Beathard G. A., Lewis S. R., Lynch J. B., Ritzmann S. E. Mixed lymphocyte culture reaction in patients with acute thermal burns. J Trauma. 1974 Jan;14(1):53–57. doi: 10.1097/00005373-197401000-00007. [DOI] [PubMed] [Google Scholar]
- Slade M. S., Simmons R. L., Yunis E., Greenberg L. J. Immunodepression after major surgery in normal patients. Surgery. 1975 Sep;78(3):363–372. [PubMed] [Google Scholar]
- Smith J. W., Steiner A. L., Parker C. W. Human lymphocytic metabolism. Effects of cyclic and noncyclic nucleotides on stimulation by phytohemagglutinin. J Clin Invest. 1971 Feb;50(2):442–448. doi: 10.1172/JCI106511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane J. R. The release and fate of vaso-active hormones in the circulation. Br J Pharmacol. 1969 Feb;35(2):209–242. doi: 10.1111/j.1476-5381.1969.tb07982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vose B. M., Moudgil G. C. Effect of surgery on tumour-directed leucocyte responses. Br Med J. 1975 Jan 11;1(5949):56–58. doi: 10.1136/bmj.1.5949.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vose B. M., Moudgil G. C. Post-operative depression of antibody-dependent lymphocyte cytotoxicity following minor surgery and anaesthesia. Immunology. 1976 Jan;30(1):123–128. [PMC free article] [PubMed] [Google Scholar]
- Webel M. L., Ritts R. E., Jr, Taswell H. F., Danadio J. V., Jr, Woods J. E. Cellular immunity after intravenous administration of methylprednisolone. J Lab Clin Med. 1974 Mar;83(3):383–392. [PubMed] [Google Scholar]


