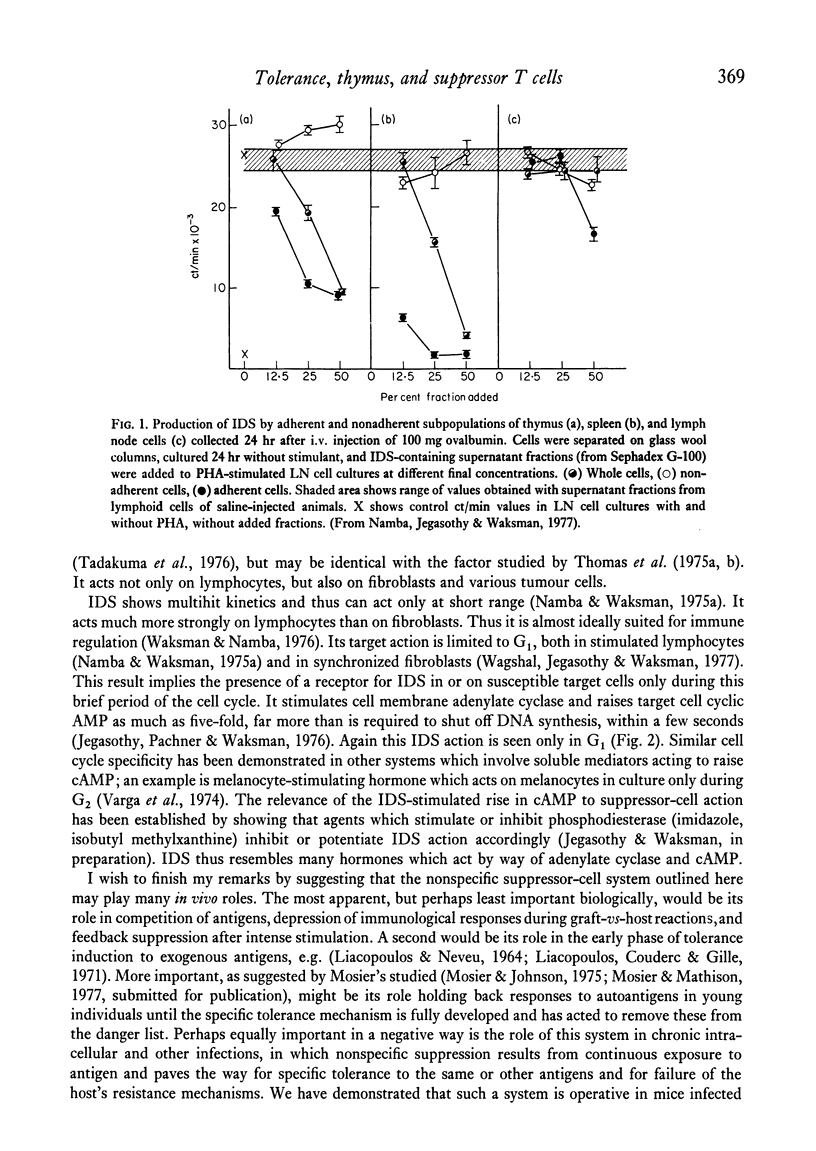
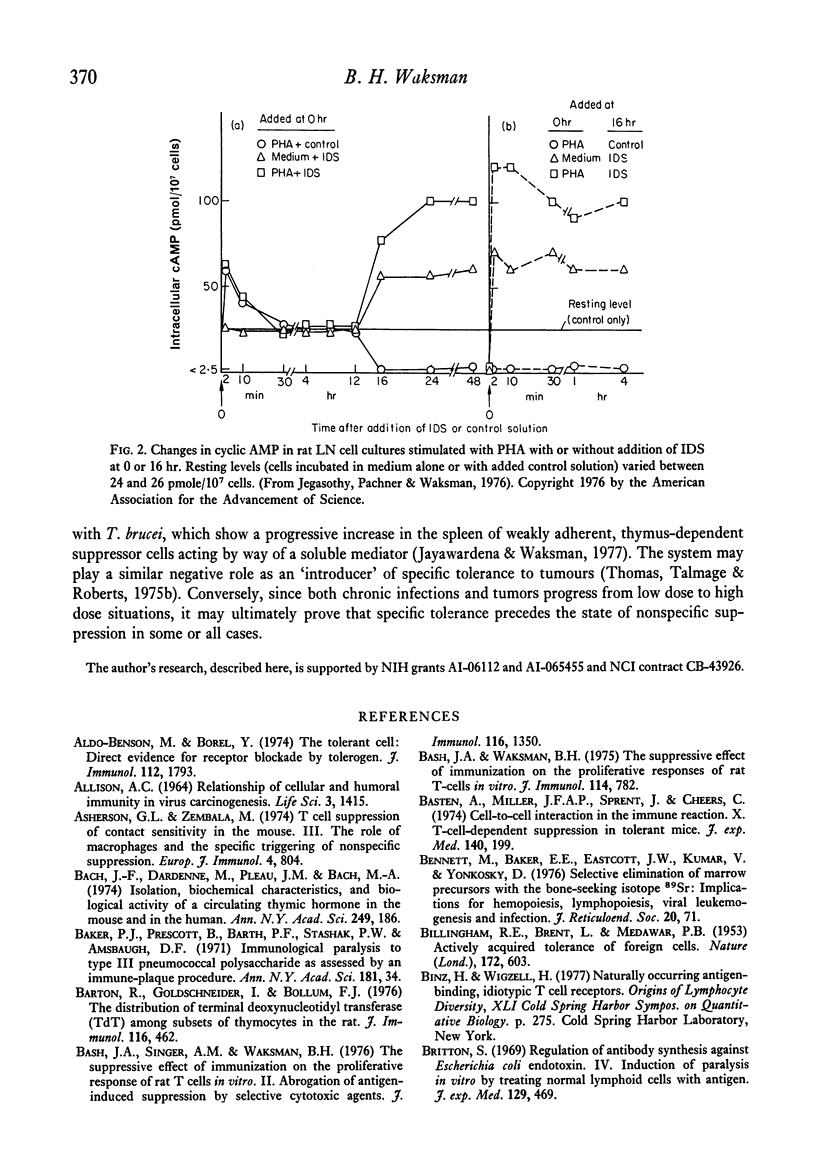
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C. RELATIONSHIP OF CELLULAR AND HUMORAL IMMUNITY IN VIRUS CARCINOGENESIS. Life Sci. 1964 Dec;3:1415–1422. doi: 10.1016/0024-3205(64)90083-9. [DOI] [PubMed] [Google Scholar]
- Aldo-Benson M., Borel Y. The tolerant cell: direct evidence for receptor blockade by tolerogen. J Immunol. 1974 May;112(5):1793–1803. [PubMed] [Google Scholar]
- BILLINGHAM R. E., BRENT L., MEDAWAR P. B. Actively acquired tolerance of foreign cells. Nature. 1953 Oct 3;172(4379):603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- Bach J. F., Dardenne M., Pleau J. M., Bach M. A. Isolation, biochemical characteristics, and biological activity of a circulating thymic hormone in the mouse and in the human. Ann N Y Acad Sci. 1975 Feb 28;249:186–210. doi: 10.1111/j.1749-6632.1975.tb29068.x. [DOI] [PubMed] [Google Scholar]
- Barton R., Goldschneider I., Bollum F. J. The distribution of terminal deoxynucleotidyl transferase (TdT) among subsets of thymocytes in the rat. J Immunol. 1976 Feb;116(2):462–468. [PubMed] [Google Scholar]
- Bash J. A., Singer A. M., Waksman B. H. The suppressive effect of immunization on the proliferative responses of rat T cells in vitro. II. Abrogation of antigen-induced suppression by selective cytotoxic agents. J Immunol. 1976 May;116(5):1350–1353. [PubMed] [Google Scholar]
- Bash J. A., Waksman B. H. The suppressive effect of immunization on the proliferative responses of rat T cells in vitro. J Immunol. 1975 Feb;114(2 Pt 2):782–787. [PubMed] [Google Scholar]
- Basten A., Miller J. F., Sprent J., Cheers C. Cell-to-cell interaction in the immune response. X. T-cell-dependent suppression in tolerant mice. J Exp Med. 1974 Jul 1;140(1):199–217. doi: 10.1084/jem.140.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M., Baker E. E., Eastcott J. W., Kumar V., Yonkosky D. Selective elimination of marrow precursors with the bone-seeking isotope 89Sr: implications for hemopoiesis, lymphopoiesis, viral leukemogenesis and infection. J Reticuloendothel Soc. 1976 Jul;20(1):71–87. [PubMed] [Google Scholar]
- Binz H., Wigzell H. Antigen-binding, idiotypic receptors from T lymphocytes: an analysis of their biochemistry, genetics, and use as immunogens to produce specific immune tolerance. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):275–284. doi: 10.1101/sqb.1977.041.01.033. [DOI] [PubMed] [Google Scholar]
- Britton S. Regulation of antibody synthesis against Escherichia coli endotoxin. IV. Induction of paralysis in vitro by treating normal lymphoid cells with antigen. J Exp Med. 1969 Mar 1;129(3):469–482. doi: 10.1084/jem.129.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975 Jun 1;141(6):1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Shen F. W., Boyse E. A. Separation of helper T cells from suppressor T cells expressing different Ly components. II. Activation by antigen: after immunization, antigen-specific suppressor and helper activities are mediated by distinct T-cell subclasses. J Exp Med. 1976 Jun 1;143(6):1391–1340. doi: 10.1084/jem.143.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess L., Evans R., Humphreys R. E., Strominger J. L., Schlossman S. F. Inhibition of antibody-dependent cellular cytotoxicity and immunoglobulin synthesis by an antiserum prepared against a human B-cell Ia-like molecule. J Exp Med. 1976 Jul 1;144(1):113–122. doi: 10.1084/jem.144.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. G., Ada G. L. Delayed-type hypersensitivity in the mouse. 3. Inactivation of thymus-derived effector cells and their precursors. Scand J Immunol. 1972;1(3):247–253. doi: 10.1111/j.1365-3083.1972.tb01816.x. [DOI] [PubMed] [Google Scholar]
- DIETRICH F. M., WEIGLE W. O. IMMUNOLOGIC UNRESPONSIVENESS TO HETEROLOGOUS SERUM PROTEINS INDUCED IN ADULT MICE AND TRANSFER OF THE UNRESPONSIVE STATE. J Immunol. 1964 Feb;92:167–172. [PubMed] [Google Scholar]
- Desaymard C., Pearce B., Feldmann M. Role of epitope density in the induction of tolerance and immunity with thymus-independent antigens. III. Interaction of epitope density and receptor avidity. Eur J Immunol. 1976 Sep;6(9):646–650. doi: 10.1002/eji.1830060910. [DOI] [PubMed] [Google Scholar]
- Diener E., Armstrong W. D. Induction of antibody formation and tolerance in vitro to a purified protein antigen. Lancet. 1967 Dec 16;2(7529):1281–1285. doi: 10.1016/s0140-6736(67)90394-7. [DOI] [PubMed] [Google Scholar]
- Diener E., Feldmann M. Antibody-mediated suppression of the immune response in vitro. II. A new approach to the phenomenon of immunological tolerance. J Exp Med. 1970 Jul 1;132(1):31–43. doi: 10.1084/jem.132.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener E., Feldmann M. Relationship between antigen and antibody-induced suppression of immunity. Transplant Rev. 1972;8:76–103. doi: 10.1111/j.1600-065x.1972.tb01565.x. [DOI] [PubMed] [Google Scholar]
- Durkin H. G., Bash J. A., Waksman B. H. Separation of T cell subpopulations capable of DNA synthesis, lymphotoxin release, and regulation of antigen and phytohemagglutinin responses on the basis of density and adherence properties. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5090–5094. doi: 10.1073/pnas.72.12.5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichmann K. Idiotype suppression. II. Amplification of a suppressor T cell with anti-idiotypic activity. Eur J Immunol. 1975 Aug;5(8):511–517. doi: 10.1002/eji.1830050802. [DOI] [PubMed] [Google Scholar]
- Feldmann M., Beverley P., Erb P., Howie S., Kontiainen S., Maoz A., Mathies M., McKenzie I., Woody J. Current concepts of the antibody response: heterogeneity of lymphoid cells, interactions, and factors. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):113–118. doi: 10.1101/sqb.1977.041.01.015. [DOI] [PubMed] [Google Scholar]
- Feldmann M., Howard J. G., Desaymard C. Role of antigen structure in the discrimination between tolerance and immunity by b cells. Transplant Rev. 1975;23:78–97. doi: 10.1111/j.1600-065x.1975.tb00150.x. [DOI] [PubMed] [Google Scholar]
- Fitch F. W. Selective suppression of immune responses. Regulation of antibody formation and cell-mediated immunity by antibody. Prog Allergy. 1975;19:195–244. [PubMed] [Google Scholar]
- Folch H., Waksman B. H. In vitro responses of rat lymphocytes following adult thymectomy. I. Rapid decrease and recovery of responses to mitogens and hemiallogeneic cells. J Immunol. 1972 Nov;109(5):1046–1051. [PubMed] [Google Scholar]
- Folch H., Waksman B. H. In vitro responses of rat lymphocytes following adult thymectomy. II. Increased inhibition by splenic adherent cells of responses to phytohemagglutinin. Cell Immunol. 1973 Oct;9(1):25–31. doi: 10.1016/0008-8749(73)90164-0. [DOI] [PubMed] [Google Scholar]
- Folch H., Waksman B. H. Regulation of lymphocyte responses in vitro. V. Suppressor activity of adherent and nonadherent rat lymphoid cells. Cell Immunol. 1973 Oct;9(1):12–24. doi: 10.1016/0008-8749(73)90163-9. [DOI] [PubMed] [Google Scholar]
- Folch H., Waksman B. H. The splenic suppressor cell. I. Activity of thymus-dependent adherent cells: changes with age and stress. J Immunol. 1974 Jul;113(1):127–139. [PubMed] [Google Scholar]
- Folch H., Waksman B. H. The splenic suppressor cell. II. Suppression of mixed lymphocyte reaction by thymus-dependent adherent cells. J Immunol. 1974 Jul;113(1):140–144. [PubMed] [Google Scholar]
- Folch H., Yoshinaga M., Waksman B. H. Regulation of lymphocyte responses in vitro. 3. Inhibition by adherent cells of the T-lymphocyte response to phytohemagglutinin. J Immunol. 1973 Mar;110(3):835–839. [PubMed] [Google Scholar]
- Follett D. A., Battisto J. R., Bloom B. R. Tolerance to a defined chemical hapten produced in adult guinea-pigs after thymectomy. Immunology. 1966 Jul;11(1):73–76. [PMC free article] [PubMed] [Google Scholar]
- Fujimoto S., Greene M. I., Sehon A. H. Regualtion of the immune response to tumor antigens. I. Immunosuppressor cells in tumor-bearing hosts. J Immunol. 1976 Mar;116(3):791–799. [PubMed] [Google Scholar]
- Fujimoto S., Greene M. I., Sehon A. H. Regulation of the immune response to tumor antigens. II. The nature of immunosuppressor cells in tumor-bearing hosts. J Immunol. 1976 Mar;116(3):800–806. [PubMed] [Google Scholar]
- Gershon R. K. A disquisition on suppressor T cells. Transplant Rev. 1975;26:170–185. doi: 10.1111/j.1600-065x.1975.tb00179.x. [DOI] [PubMed] [Google Scholar]
- Gershon R. K., Gery I., Waksman B. H. Suppressive effects of in vivo immunization on PHA responses in vitro. J Immunol. 1974 Jan;112(1):215–221. [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970 May;18(5):723–737. [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K., Kondo K. Infectious immunological tolerance. Immunology. 1971 Dec;21(6):903–914. [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K. T cell control of antibody production. Contemp Top Immunobiol. 1974;3:1–40. doi: 10.1007/978-1-4684-3045-5_1. [DOI] [PubMed] [Google Scholar]
- Gery I., Waksman B. H. Role of the thymus in tolerance. V. Suppressive effect of treatment with nonaggregated and aggregated bovine gamma-globulin on specific immune responses in normal adult rats. J Immunol. 1967 Mar;98(3):446–460. [PubMed] [Google Scholar]
- Godfrey H. P. The use of 1-fluoro-2,4-dinitrobenzene as an affinity label for the antigen receptor of delayed hypersensitivity. Immunology. 1976 Oct;31(4):665–673. [PMC free article] [PubMed] [Google Scholar]
- Greenberg A. H., Playfair J. H. Spontaneously arising cytotoxicity to the P-815-Y mastocytoma in NZB mice. Clin Exp Immunol. 1974 Jan;16(1):99–109. [PMC free article] [PubMed] [Google Scholar]
- Greenberg A. H., Shen L., Walker L., Arnaiz-Villena A., Roitt I. M. Characteristics of the effector cells mediating cytotoxicity against antibody-coated target cells. II. The mouse nonadherent K cell. Eur J Immunol. 1976 Jul;5(7):474–480. doi: 10.1002/eji.1830050709. [DOI] [PubMed] [Google Scholar]
- Ha T. Y., Waksman B. H. Role of the thymus in tolerance. X. "Suppressor" activity of antigen-stimulated rat thymocytes transferred to normal recipients. J Immunol. 1973 May;110(5):1290–1299. [PubMed] [Google Scholar]
- Ha T. Y., Waksman B. H., Treffers H. P. The thymic suppre-sor cell. II. Metabolic requirements of suppressor activity. Immunol Commun. 1974;3(4):351–359. doi: 10.3109/08820137409061115. [DOI] [PubMed] [Google Scholar]
- Ha T. Y., Waksman B. H., Treffers H. P. The thymic suppressor cell. I. Separation of subpopulations with suppressor activity. J Exp Med. 1974 Jan 1;139(1):13–23. doi: 10.1084/jem.139.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström K. E., Hellström I. Lymphocyte-mediated cytotoxicity and blocking serum activity to tumor antigens. Adv Immunol. 1974;18:209–277. doi: 10.1016/s0065-2776(08)60311-9. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Lavrin D. H., Asofsky R. Effect of antibody to theta antigen on cell-mediated immunity induced in syngeneic mice by murine sarcoma virus. J Natl Cancer Inst. 1973 Nov;51(5):1509–1512. doi: 10.1093/jnci/51.5.1509. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Herzenberg L. A. Short-term and chronic allotype suppression in mice. Contemp Top Immunobiol. 1974;3:41–75. doi: 10.1007/978-1-4684-3045-5_2. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Okumura K., Cantor H., Sato V. L., Shen F. W., Boyse E. A., Herzenberg L. A. T-cell regulation of antibody responses: demonstration of allotype-specific helper T cells and their specific removal by suppressor T cells. J Exp Med. 1976 Aug 1;144(2):330–344. doi: 10.1084/jem.144.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi A., Gery I., Waksman B. H. Role of the thymus in tolerance. VII. Distribution of nonaggregated and heat-aggregated bovine gamma globulin in lymphoid organs of normal newborn and adult rats. Yale J Biol Med. 1968 Aug;41(1):13–32. [PMC free article] [PubMed] [Google Scholar]
- Horiuchi A., Waksman B. H. Role of the thymus in tolerance. 8. Relative effectiveness of nonaggregated and heat-aggregated bovine gamma globulin, injected directly into lymphoid organs of normal rats, in suppressing immune responsiveness. J Immunol. 1968 Dec;101(6):1322–1332. [PubMed] [Google Scholar]
- Horiuchi A., Waksman B. H. Role of the thymus in tolerance. VI. Tolerance to bovine gamma-globulin in rats given a low dose of irradiation and injection of nonaggregated or aggregated antigen into the shielded thymus. J Immunol. 1968 May;100(5):974–978. [PubMed] [Google Scholar]
- Howard J. G., Mitchison N. A. Immunological tolerance. Prog Allergy. 1975;18:43–96. doi: 10.1159/000395256. [DOI] [PubMed] [Google Scholar]
- Huber B., Cantor H., Shen F. W., Boyse E. A. Independent differentiative pathways of Ly1 and Ly23 subclasses of T cells. Experimental production of mice deprived of selected T-cell subclasses. J Exp Med. 1976 Oct 1;144(4):1128–1133. doi: 10.1084/jem.144.4.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorio R. J., Chanana A. D., Cronkite E. P., Joel D. D. Studies on lymphocytes. XVI. Distribution of bovine thymic lymphocytes in the spleen and lymph nodes. Cell Tissue Kinet. 1970 Apr;3(2):161–173. [PubMed] [Google Scholar]
- Isaković K., Smith S. B., Waksman B. H. Role of the thymus in tolerance. I. Tolerance to bovine gamma globulin in thymectomized, irradiated rats grafted with thymus from tolerant donors. J Exp Med. 1965 Dec 1;122(6):1103–1123. doi: 10.1084/jem.122.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson E. B., Herzenberg L. A., Riblet R., Herzenberg L. A. Active suppression of immunoglobulin allotype synthesis. II. Transfer of suppressing factor with spleen cells. J Exp Med. 1972 May 1;135(5):1163–1176. doi: 10.1084/jem.135.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsson H., Blomgren H. Changes of the PHA-responding pool of cells in the thymus after cortisone or x-ray treatment of mice. Evidence for an invese relation between the production of cortical and medullary thymocytes. Cell Immunol. 1972 May;4(1):93–105. doi: 10.1016/0008-8749(72)90008-1. [DOI] [PubMed] [Google Scholar]
- Jayawardena A. N., Waksman B. H. Suppressor cells in experimentally trypanosomiasis. Nature. 1977 Feb 10;265(5594):539–541. doi: 10.1038/265539a0. [DOI] [PubMed] [Google Scholar]
- Jegasothy B. V., Pachner A. R., Waksman B. H. Cytokine inhibition of DNA synthesis: effect on cyclic adenosine monophosphate in lymphocytes. Science. 1976 Sep 24;193(4259):1260–1262. doi: 10.1126/science.183266. [DOI] [PubMed] [Google Scholar]
- Kapp J. A., Pierce C. W., De la Croix F., Benacerraf B. Immunosuppressive factor(s) extracted from lymphoid cells of nonresponder mice primed with L-glutamic acid60-L-alanine30-L-tyrosine10 (GAT). J Immunol. 1976 Feb;116(2):305–309. [PubMed] [Google Scholar]
- Kapp J. A., Pierce C. W., Schlossman S., Benacerraf B. Genetic control of immune responses in vitro. V. Stimulation of suppressor T cells in nonresponder mice by the terpolymer L-glutamic acid 60-L-alanine 30-L-tyrosine 10 (GAT). J Exp Med. 1974 Sep 1;140(3):648–659. doi: 10.1084/jem.140.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Hunter P. C., Jacobs D., Lord E. Functional heterogeneity among the T-derived lymphocytes of the mouse. I. Analysis by adult thymectomy. J Immunol. 1974 Jul;113(1):27–38. [PubMed] [Google Scholar]
- Katz D. H., Davie J. M., Paul W. E., Benacerraf B. Carrier function in anti-hapten antibody responses. IV. Experimental conditions for the induction of hapten-specific tolerance or for the stimulation of anti-hapten anamnestic responses by "nonimmunogenic" hapten-polypeptide conjugates. J Exp Med. 1971 Jul 1;134(1):201–223. doi: 10.1084/jem.134.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Pross H., Wigzell H. "Natural" killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol. 1975 Feb;5(2):117–121. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- LIACOPOULOS P., NEVEU T. NON-SPECIFIC INHIBITION OF THE IMMEDIATE AND DELAYED TYPES OF HYPERSENSITIVITY DURING IMMUNE PARALYSIS OF ADULT GUINEA-PIGS. Immunology. 1964 Jan;7:26–39. [PMC free article] [PubMed] [Google Scholar]
- Lamon E. W., Skurzak H. M., Klein E., Wigzell H. In vitro cytotoxicity by a nonthymus-processed lymphocyte population with specificity for a virally determined tumor cell surface antigen. J Exp Med. 1972 Nov 1;136(5):1072–1079. doi: 10.1084/jem.136.5.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton A. R., 3rd, Cooper M. D. Modification of B lymphocyte differentiation by anti-immunoglobulins. Contemp Top Immunobiol. 1974;3:193–225. doi: 10.1007/978-1-4684-3045-5_8. [DOI] [PubMed] [Google Scholar]
- Liacopoulos P., Couderc J., Gille M. F. Competition of antigens during induction of low zone tolerance. Eur J Immunol. 1971 Nov;1(5):359–363. doi: 10.1002/eji.1830010511. [DOI] [PubMed] [Google Scholar]
- Loor F., Amstutz H., Hägg L. B., Mayor K. S., Roelants G. E. T lineage lymphocytes in nude mice born from homozygous nu/nu parents. Eur J Immunol. 1976 Sep;6(9):663–665. doi: 10.1002/eji.1830060914. [DOI] [PubMed] [Google Scholar]
- Lotzová E., Gallagher M. T., Trentin J. J. Macrophage involvement in genetic resistance to bone marrow transplantation. Transplant Proc. 1976 Sep;8(3):477–482. [PubMed] [Google Scholar]
- MARSHALL A. H., WHITE R. G. The immunological reactivity of the thymus. Br J Exp Pathol. 1961 Aug;42:379–385. [PMC free article] [PubMed] [Google Scholar]
- MOLLER G., MOLLER E. Studies in vitro and in vivo of the cytotoxic and enhancing effect of humoral isoantibodies. Ann N Y Acad Sci. 1962 Oct 24;99:504–530. doi: 10.1111/j.1749-6632.1962.tb45332.x. [DOI] [PubMed] [Google Scholar]
- Metcalf E. S., Klinman N. R. In vitro tolerance induction of neonatal murine B cells. J Exp Med. 1976 Jun 1;143(6):1327–1340. doi: 10.1084/jem.143.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier D. E., Johnson B. M. Ontogeny of mouse lymphocyte function. II. Development of the ability to produce antibody is modulated by T lymphocytes. J Exp Med. 1975 Jan 1;141(1):216–226. doi: 10.1084/jem.141.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba Y., Waksman B. H. Regulatory substances produced by lymphocytes--IV. Further characterization of the inhibitor of DNA synthesis (IDS). Immunochemistry. 1977 Feb;14(2):143–147. doi: 10.1016/0019-2791(77)90293-2. [DOI] [PubMed] [Google Scholar]
- Namba Y., Waksman B. H. Regulatory substances produced by lymphocytes. II. Lymphotoxin in the rat. J Immunol. 1975 Oct;115(4):1018–1022. [PubMed] [Google Scholar]
- Namba Y., Waksman B. H. Regulatory substances produced by lymphocytes. III. Evidence that lymphotoxin and proliferation inhibitory factor are identical and different from the inhibitor of DNA synthesis. J Immunol. 1976 Apr;116(4):1140–1144. [PubMed] [Google Scholar]
- Nossal G. J., Pike B. L. Evidence for the clonal abortion theory of B-lymphocyte tolerance. J Exp Med. 1975 Apr 1;141(4):904–917. [PMC free article] [PubMed] [Google Scholar]
- Owen R. D. IMMUNOGENETIC CONSEQUENCES OF VASCULAR ANASTOMOSES BETWEEN BOVINE TWINS. Science. 1945 Oct 19;102(2651):400–401. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- Penfold P. L., Greenberg A. H., Roitt I. M. Characteristics of the effector cells mediating cytotoxicity against antibody-coated target cells. III. Ultrastructural studies. Clin Exp Immunol. 1976 Jan;23(1):91–97. [PMC free article] [PubMed] [Google Scholar]
- Pierce C. W., Kapp J. A. Regulation of immune responses by suppressor T cells. Tohoku J Exp Med. 1976;5:91–143. [PubMed] [Google Scholar]
- Pierce C. W., Solliday S. M., Asofsky R. Immune responses in vitro. IV. Suppression of primary M, G, and A plaque-forming cell responses in mouse spleen cell cultures by class-specific antibody to mouse immunoglobulins. J Exp Med. 1972 Mar 1;135(3):675–697. doi: 10.1084/jem.135.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Owen J. J., Cooper M. D., Lawton A. R., 3rd, Megson M., Gathings W. E. Differences in susceptibility of mature and immature mouse B lymphocytes to anti-immunoglobulin-induced immunoglobulin suppression in vitro. Possible implications for B-cell tolerance to self. J Exp Med. 1975 Nov 1;142(5):1052–1064. doi: 10.1084/jem.142.5.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelants G. E., Mayor K. S., Hägg L. B., Loor F. Immature T lineage lymphocytes in athymic mice. Presence of TL, lifespan and homeostatic regulation. Eur J Immunol. 1976 Feb;6(2):75–81. doi: 10.1002/eji.1830060202. [DOI] [PubMed] [Google Scholar]
- Ruuskanen O. Subpopulations of Guinea pig thymocytes. Different distribution patterns of alkaline phosphatase positive and negative autologous thymocytes. Cell Immunol. 1975 Feb;15(2):246–254. doi: 10.1016/0008-8749(75)90003-9. [DOI] [PubMed] [Google Scholar]
- Schlesinger M., Israël E. The recovery of spleen-seeking and lymph-node-seeking thymus subpopulations following cortisol administration. Cell Immunol. 1975 Jul;18(1):144–151. doi: 10.1016/0008-8749(75)90043-x. [DOI] [PubMed] [Google Scholar]
- Scott D. W., Waksman B. H. Mechanism of immunologic tolerance. I. Induction of tolerance to bovine gamma-globulin by injection of antigen into intact organs in vitro. J Immunol. 1969 Feb;102(2):347–354. [PubMed] [Google Scholar]
- Scott D. W., Waksman B. H. Tolerance in vitro: suppression of immune responsiveness to bovine gamma-globulin after injection of antigen into intact lymphoid organs. J Immunol. 1968 Apr;100(4):912–914. [PubMed] [Google Scholar]
- Shearer G. M., Garbarino C. A., Cudkowicz G. In vitro induction of F1 hybrid anti-parent cell-mediated cytotoxicity. J Immunol. 1976 Sep;117(3):754–759. [PubMed] [Google Scholar]
- Shortman K., Jackson H. The differentiation of T lymphocytes. I. Proliferation kinetics and interrelationships of subpopulations of mouse thymus cells. Cell Immunol. 1974 May;12(2):230–246. doi: 10.1016/0008-8749(74)90075-6. [DOI] [PubMed] [Google Scholar]
- Shortman K., Von Boehmer H., Lipp J., Hopper K. Subpopulations of T-lymphocytes. Physical separation, functional specialisation and differentiation pathways of sub-sets of thymocytes and thymus-dependent peripheral lymphocytes. Transplant Rev. 1975;25:163–210. [PubMed] [Google Scholar]
- Smith J. B., Eaton G. J. Suppressor cells in spleens from "nude" mice: their effect on the mitogenic response of B lymphocytes. J Immunol. 1976 Jul;117(1):319–322. [PubMed] [Google Scholar]
- Smith S. B., Isakovic K., Waksman B. H. Role of the thymus in tolerance. II. Transfer of specific unresponsiveness to BSA with thymus grafting. Proc Soc Exp Biol Med. 1966 Apr;121(4):1005–1008. doi: 10.3181/00379727-121-30949. [DOI] [PubMed] [Google Scholar]
- Staples P. J., Gery I., Waksman B. H. Role of the thymus in tolerance. 3. Tolerance to bovine gamma globulin after direct injection of antigen into the shielded thymus of irradiated rats. J Exp Med. 1966 Aug 1;124(2):127–139. doi: 10.1084/jem.124.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutman O. Humoral thymic factors influencing postthymic cells. Ann N Y Acad Sci. 1975 Feb 28;249:89–105. doi: 10.1111/j.1749-6632.1975.tb29060.x. [DOI] [PubMed] [Google Scholar]
- Tada T. Regulation of reaginic antibody formation in animals. Prog Allergy. 1975;19:122–194. [PubMed] [Google Scholar]
- Tada T., Taniguchi M., David C. S. Suppressive and enhancing T-cell factors as I-region gene products: properties and the subregion assignment. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):119–127. doi: 10.1101/sqb.1977.041.01.016. [DOI] [PubMed] [Google Scholar]
- Tada T., Taniguchi M., Takemori T. Properties of primed suppressor T cells and their products. Transplant Rev. 1975;26:106–129. doi: 10.1111/j.1600-065x.1975.tb00177.x. [DOI] [PubMed] [Google Scholar]
- Tadakuma T., Kühner A. L., Rich R. R., David J. R., Pierce C. W. Biological expressions of lymphocyte activation. V. Characterization of a soluble immune response suppressor (SIRS) produced by concanavalin A-activated spleen cells. J Immunol. 1976 Jul;117(1):323–330. [PubMed] [Google Scholar]
- Thomas D. W., Roberts W. K., Talmage D. W. Regulation of the immune response: production of a soluble suppressor by immune spleen cells in vitro. J Immunol. 1975 May;114(5):1616–1622. [PubMed] [Google Scholar]
- Thomas D. W., Talmage D. W., Joberts W. K. Inhibiton of tumor cell proliferation: second role for suppressor cells? J Immunol. 1975 Nov;115(5):1366–1374. [PubMed] [Google Scholar]
- Toullet F. T., Waksman B. H. Role of the thymus in tolerance. IV. Specific tolerance to homografts in neonatally thymectomized mice grafted with thymus from tolerant donors. J Immunol. 1966 Nov;97(5):686–692. [PubMed] [Google Scholar]
- Varga J. M., Dipasquale A., Pawelek J., McGuire J. S., Lerner A. B. Regulation of melanocyte stimulating hormone action at the receptor level: discontinuous binding of hormone to synchronized mouse melanoma cells during the cell cycle. Proc Natl Acad Sci U S A. 1974 May;71(5):1590–1593. doi: 10.1073/pnas.71.5.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisin G. A. Immunological facilitation, a broadening of the concept of the enhancement phenomenon. Prog Allergy. 1971;15:328–485. [PubMed] [Google Scholar]
- Vojtisková M., Lengerová A. On the possibility that thymus-mediated alloantigenic stimulation results in tolerance response. Experientia. 1965 Nov 15;21(11):661–663. doi: 10.1007/BF02144067. [DOI] [PubMed] [Google Scholar]
- Waksman B. H., Namba Y. On soluble mediators of immunologic regulation. Cell Immunol. 1976 Jan;21(1):161–176. doi: 10.1016/0008-8749(76)90337-3. [DOI] [PubMed] [Google Scholar]


