Abstract
Changes of membrane lipids in cisplatin-sensitive A549 and cisplatin-resistant A549/DDP cells during the apoptotic process induced by a clinical dose of cisplatin (30 μM) were detected by 1H and 31P-NMR spectroscopy and by membrane fluidity measurement. The apoptotic phenotypes of the two cell lines were monitored with flow cytometry. The assays of apoptosis showed that significant apoptotic characteristics of the A549 cells were induced when the cells were cultured for 24 hours after treatment with cisplatin, while no apoptotic characteristic could be detected for the resistant A549/DDP cells even after 48 hours. The results of 1H-NMR spectroscopy demonstrated that the CH2/CH3 and Glu/Ct ratios of the membrane of A549 cells increased significantly, but those in A549/DDP cell membranes decreased. In addition, the Chol/CH3 and Eth/Ct ratios decreased for the former but increased for the latter cells under the same conditions. 31P-NMR spectroscopy indicated levels of phosphomonoesters (PME) and ATP decreased in A549 but increased in A549/DDP cells after being treated with cisplatin. These results were supported with the data obtained from 1H-NMR measurements. The results clearly indicated that components and properties of membrane phospholipids of the two cell lines were significantly different during the apoptotic process when they were treated with a clinical dose of cisplatin. Plasma membrane fluidity changes during cisplatin treatment as detected with the fluorescence probe TMA-DPH also indicate marked difference between the two cell lines. We provided evidence that there are significant differences in plasma membrane changes during treatment of cisplatin sensitive A549 and resistant A549/DDP cells.
Keywords: A549 cells, cisplatin, membrane lipids, 1H-NMR, 31P-NMR
Background
Chemotherapy can cure many human cancers, but drug resistance – intrinsic or acquired – is a major problem for many patients. Many changes are associated with multi-drug resistance (MDR) of tumor cells [1,2]. One of them, alterations of drug accumulation and distribution in cells seem to play an important role in MDR [3]. Efflux pumps, such as, P-glycoprotein, MRP (multi-drug resistance protein), and LRP (lung resistant protein) can decrease drug accumulation in cells and make chemotherapy ineffective [4]. The plasma membrane plays an important role in the control of intracellular concentration, efflux and influx of drugs. Many anticancer drugs show membrane effects via weak hydrophobic interaction or via electrostatic binding to membrane phospholipids before entering the cytoplasm. Adriamycin, for example, interacts with the membrane by increasing the turnover of phosphatidylinositol [5], and cisplatin can interact with phosphatidylserine to alter the characteristic of the membrane [6]. Remarkably, in tumor cells, the development of drug resistance usually has been associated with changes in their plasma membrane phospholipids. For example, daunomycin-resistance in P338 cells [7] is associated with fewer drugs retained in resistant cells than in their sensitive counterparts and the resistant cells were shown to have a lower phosphatidylserine content and a higher cholesterol content in their plasma membrane than sensitive cells. The development of vinblastine resistance in human leukemic lymphoblast was associated with an increase in cholesterol and phopholipids content in their membranes [8]. In Ehrlich ascites tumor cells, resistance was reported to depend on the level of phospholipids in the plasma membrane [9]. These observations demonstrate the relationship between drug resistant phenotypes and the changes of the components and properties of the plasma membrane lipids.
Fluorescence measurement is a method used to detect the physical state changes of membrane lipids. Recently, 1H-NMR and 31P-NMR spectra have also been used in detecting changes of membrane lipids. NMR provides a rapid, accurate, and noninvasive means particularly useful for detection of changes in membrane lipids in living cells [10]. Physical state differences between human lung adenocarcinoma cisplatin sensitive A549 and cisplatin resistant A549/DDP cells were reported previously by fluorescence measurement in our laboratory [11]. Presently, we have extended this study, by following the apoptosis process and plasma membrane fatty composition of the cisplatin sensitive A549 and resistant A549/DDP cells after treatment with a clinical dose of cisplatin.
Results
Apoptotic characteristics of A549/DDP cells
Recently, flow cytometry has been applied to study DNA changes of cellular population [12] in order to detect cell apoptotic characteristics. Fig. 1 shows the apoptotic peak of the two cell lines monitored by flow cytometry. It can be seen that the sub-G1 phase of the sensitive A549 cells appeared after 24 h culture of the cells [Fig. 1(b)], but there is no sub-G1 phase of the resistant A549/DDP cells even after 48 h culture [Fig. 1(f)]. Apoptotic cells of the sensitive A549 increased from 55% at 24 h to 72% at 48 h compared to the control in sensitive A549 cells [Fig. 1(a)].
Figure 1.
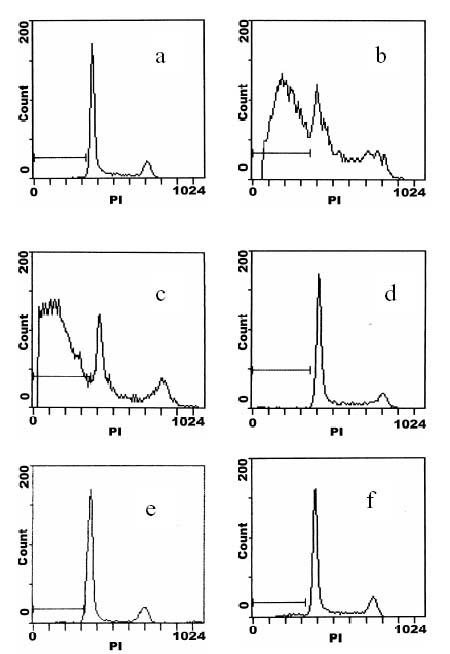
Flow cytometry histograms of A549 and A549/DDP cells treated with a clinical dose of 30 μM cisplatin. a, b, c stands for A549 cells treated and cultured for 0, 24, and 48 h, respectively, and d, e, f for A549/DDP cells under the same conditions.
The 1H-NMR spectra of sensitive A549 and resistant A549/DDP cells
The 1D 1H-NMR spectra of A549 and A549/DDP cells are showed in Fig. 2(A) and Fig. 2(B), respectively. Table 1 lists the ratios of the corresponding peak intensities. The changes in composition and properties of the membrane lipids between the two cell lines can be deduced from changes of the peak intensities of fatty acids [13]. It can be seen that there is an increase in CH2/CH3 and Glu/Ct ratios but a decrease in Chol/CH3 and Eth/Ct ratios of the A549 cells during the apoptotic progress induced by cisplatin. However, it is noticeable that the changes of CH2/CH3 and Glu/Ct ratios as well as Chol/CH3 and Eth/Ct ratios of the resistant A549/DDP cells are just the opposite from those of the sensitive A549 cells.
Figure 2.
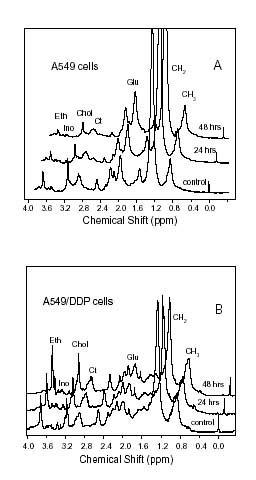
1H-NMR spectra of A549 cells (A) and A549/DDP cells (B) treated with a clinical dose of 30 μM cisplatin and cultured for 0 h, 24 h, and 48 h. The cell concentrations are comparable (1 × 107 cells/mL) for each sample. The peak assignments are shown in the figures. Number of scans was 128.
Table 1.
Peak intensity ratios of 1H NMR spectra obtained from A549 and A549/DDP cells treated with 30 μM cisplatin and cultured for 0 h, 24 h, and 48 h.
| Cells | Treated time | CH2/CH3 | Chol/CH3 | Glu/Ct | Eth/Ct | Ino/Ct |
| A549 | 0 hrs | 3.63 ± 0.08 | 0.26 ± 0.06 | 4.11 ± 0.15 | 0.56 ± 0.05 | 0.23 ± 0.07 |
| 24 hrs | 4.12 ± 0.09 | 0.19 ± 0.05 | 4.39 ± 0.18 | 0.41 ± 0.06 | 0.16 ± 0.06 | |
| 48 hrs | 4.25 ± 0.06 | 0.11 ± 0.06 | 4.94 ± 0.12 | 0.33 ± 0.05 | 0.12 ± 0.08 | |
| A549/DDP | 0 hrs | 2.21 ± 0.09 | 0.28 ± 0.06 | 3.66 ± 0.27 | 0.68 ± 0.04 | 0.25 ± 0.04 |
| 24 hrs | 2.04 ± 0.06 | 0.33 ± 0.04 | 3.62 ± 0.16 | 0.74 ± 0.06 | 0.29 ± 0.08 | |
| 48 hrs | 1.66 ± 0.08 | 0.39 ± 0.09 | 3.60 ± 0.03 | 0.86 ± 0.05 | 0.33 ± 0.06 | |
Data represent mean values ± SD (n = 3). P < 0.05.
The 31P-NMR spectra of sensitive A549 and resistant A549/DDP cells
Typical 31P-NMR spectra of A549 and A549/DDP cells obtained at 22°C are shown in Fig. 3(A) and 3(B), respectively. The resonance at 18 ppm is from 10 mM methylene-diphosphonate used as external concentration standard. Prominent peaks are observed for phosphomonoesters (PME), primarily from phosphoryl-ethanolamine (PE) and phosphorylcholine (PC); Pi, α-ATP; β-ATP; γ-ATP; and nicotinamide adenine dinucleotide (NAD), as well as uridine diphosphoglucose (UDPG) [14]. The signal corresponding to β-ATP was used for quantitation of all ATP resonances since there is no overlap of β-ATP resonance with other 31P signals. Changes of phosphorus metabolites of the two cell lines were shown in Table 2. 31P-NMR spectra indicated that the levels of PME and ATP were reduced in A549 but enhanced slightly in A549/DDP after treatment with cisplatin.
Figure 3.
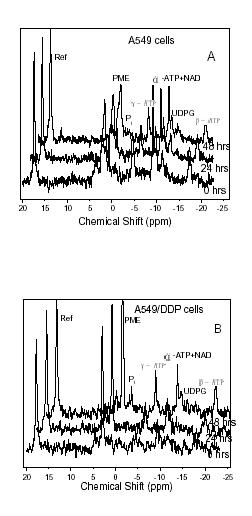
31P-NMR spectra of A549 cells (A), A549/DDP cells (B) treated with a clinical dose of 30 μM cisplatin and cultured for 0 h, 24 h, and 48 h. The cell concentrations are comparable (5 × 108 cells/mL) for each sample. The peak assignments are shown in the figures. 2000 transients were accumulated for each spectrum.
Table 2.
Comparison of the concentration of phosphorus metabolites per 5 × 108 cells at 22°C for A549 and A549/DDP cells
| Cells | Treated time | PME/Ref | γ-ATP/Ref | α-ATP+NAD/Ref | β-ATP/Ref |
| A549 | 0 hrs | 1.90 ± 0.12 | 0.76 ± 0.06 | 1.10 ± 0.05 | 0.47 ± 0.05 |
| 24 hrs | 1.25 ± 0.11 | 0.45 ± 0.05 | 0.74 ± 0.05 | 0.34 ± 0.04 | |
| 48 hrs | 1.02 ± 0.18 | 0.34 ± 0.08 | 0.65 ± 0.12 | 0.28 ± 0.05 | |
| A549/DDP | 0 hrs | 0.86 ± 0.04 | 0.57 ± 0.06 | 0.60 ± 0.05 | 0.51 ± 0.05 |
| 24 hrs | 1.04 ± 0.06 | 0.60 ± 0.05 | 0.74 ± 0.08 | 0.58 ± 0.04 | |
| 48 hrs | 1.25 ± 0.08 | 0.67 ± 0.06 | 0.87 ± 0.12 | 0.64 ± 0.05 | |
Data represent mean values ± SD (n = 3). P < 0.05.
The microviscosity of the plasma membrane of the resistant A549/DDP cells increased
A fluorescence probe TMA-DPH, which is a derivative of DPH, was used to detect the change of microviscosity of the living cells. This dye localized specifically in the plasma membrane for a sufficient time to allow plasma membrane fluidity and heterogeneity measurements of the whole cells [15]. The changes of microviscosity of the plasma membrane of A549 and A549/DDP cells are showed in Fig. 4. It is clear that microviscosity of the plasma membrane of the sensitive A549 cells induced by cisplatin decreased but that of the resistant A549/DDP treated the same increased.
Figure 4.
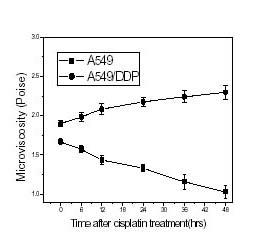
The microviscosity of the plasma membranes of A549 and A549/DDP cells treated with a clinical dose of 30 μM cisplatin and cultured for different time periods. Microviscosity was measured by the fluorescence probe TMA-DPH (2 μM) as given as <see Methods>.
Conclusion
The apoptotic characteristics of tumor cells play an important role in the development of cancer, studies on changes of plasma membrane phospholipids during the process of apoptosis of MDR tumor cells treated with chemotherapeutic drugs may indicate molecular mechanisms of the MDR phenomena. NMR spectroscopy can provide an assessment of lipid composition and changes in membrane fluidity in relation to drug resistance and apoptosis. Mountford [16] reported that, 1H-NMR spectroscopy can provide information on phospholipids in cellular membranes. Different groups in phospholipid molecules are located in microenvironments in membranes that are physical-chemically heterogeneous, and can be surveyed in 1H-NMR spectra as chemical shifts (ppm). Singh JK et al. [17] observed a significant increase in the proportion of saturated fatty acid side chains in phosphatidylserine (PS) and phosphatidylinositol when apoptosis was triggered in a clonal neuronal (hippocampal) cell line (HN2-5) by hypoxia or nutrient deprivation. Dynamic properties of plasma membrane are affected if the composition and characteristics of the phospholipids contained in the plasma membrane change. Our data clearly indicated that the peak ratios of CH2/CH3 and Glu/Ct for the sensitive A549 cells [Fig. 2(A)] increased 1.3- and 1.5-fold, respectively, compared to the resistant A549/DDP cells [Fig. 2(B)], furthermore these changes were further increased for the former during the apoptotic process induced by cisplatin, but decreased for the resistant A549/DDP (Table 1). Also, changes of the peak ratios of Chol/CH3 and Eth/Ct for the sensitive A549 cells were just the opposite from those of the resistant 549/DDP cells. These differences implied that changes in the composition of the membrane phospholipids in the two cell lines may be responsible for inducing changes of membrane dynamic characteristics, such as microviscosity or fluidity. Mountford reported that, in tumor cells, the decrease in microviscosity of the plasma membrane was related to the increase in CH2/CH3 ratio detected by 1H-NMR spectroscopy [18,19]. Our results obtained by 1H-NMR measurement are further supported by the measurement with the fluorescence dye TMA-DPH [Fig. 4].
It seems there are no experiment to support, it was suggested before, based on 31P-NMR studies that phospholipid metabolism is characteristic for tumor cells [20]; Liora [21] et al. demonstrated that there was a decrease in phosphatidylcholine level in human breast cancer cells during apoptosis due to the inhibited choline transport and the enhanced activity of phosphocholine: cytidine triphosphate cytidylyl-transferase. We speculate that the decrease of the Chol/CH3 ratio in A549 cells and the increase of the Chol/CH3 ratio in A549/DDP cells reflect biosythesis differences of phosphatidylcholine and/or the phosphocholine:cytidine triphosphate cytidylyl-transferase between A549 and A549/DDP cells. These differences may also be related to the different apoptotic characteristics of the two cell lines.
31P-NMR measurements were carried out for gaining an additional insight into the changes of phosphorus metabolism in the two cell lines after cisplatin treatment. A higher PME (PE and PC) and ATP levels were found in A549 cells in comparison with those of A549/DDP cells [Fig. 3(A), 3(B), Table 2]. A higher level of PME in some cells was considered to correlate with a higher rate of cell proliferation [22]. This may be one reason why A549 cells grow faster than A549/DDP cells. But after treatment with cisplatin, the level of PME decreased gradually in A549 but increased in A549/DDP. These results were consistent with the data obtained from 1H-NMR measurements above. In addition, the pronounced broader peak of PME of A549 cells than that of A549/DDP indicates that the head groups of the phospholipid (PE and PC) in A549 cells move slower than those of A549/DDP cells. The increase and decrease in the level of ATP in A549/DDP and A549 cells, respectively, were manifested under the same condition. The increase of PME and ATP in A549/DDP cells responding to the treatment with cisplatin would be a molecular mechanism involved in their cisplatin-resistant phenotype.
In addition to lipid signal modification, 1H-NMR spectroscopy demonstrated that the other possible metabolites were affected along with apoptosis, such as the glutamine and inositol content. Glutamine, glutamate and glutathione (GSH) are used by the cells for detoxification via glutathione conjugation. GSH covalently binding cisplatin at physiological concentration [23-25] and direct reaction between GSH and cisplatin in cells had been reported [26]. Though depletion of GSH by buthionine sulphoximine (BSO) have been ever reported not to enhance cisplatin sensitivity in some cell lines [27,28], there is considerable evidence linking GSH to cisplatin resistance [29-31] and depletion of GSH by buthionine sulphoximine (BSO) enhanced authentically cisplatin sensitivity in some cell lines [31,32]. Our data indicated that the decrease of Glu in A549/DDP [Fig. 2(A) and 2(B)], and Table (1) might be due to a higher turnover of these metabolites in the resistant cells A549/DDP in the metabolic pathway of glutamine to glutamate to glutathione, to increase glutathione conjugation. Our results are in good agreement with previous reports indicating increased glutathion-S transferate activity in resistant cells [33].
In summary, all the NMR and fluorescence anisotropy studies indicated that distinct differences of phospholipid components and properties of the plasma membrane are between sensitive A549 and resistant A549/DDP cells in the apoptotic process induced with a clinical dose of cisplatin (30 μM). We suggest that these differences play significant role in the cisplatin resistant phenotype of the A549/DDP cells.
Materials and Methods
Reagents
Cisplatin, penicillin, streptomycin, trypsin, methylene diphosphonate, percoll and 2, 2-Dimethyl-2-silapentane-5-sulfonic acid (DDS) were purchased from Sigma, and 6-diphenyl-1, 3, 5-hexatrine (TMA-DPH) and propidium iodide (PI) from Molecular Probes. Deuterium oxide was from Cambridge Isotope Laboratories, Inc. (Andover, MA, USA). Other reagents were local products of analytic grade.
Cell lines and cell culture
The parental cell line A549, a human adenocarcinoma cell line, and the cell line A549/DDP, which is resistant to cisplatin (CDDP or DDP), were generously provided by Beijing Cancer Institute, (Beijing, China.) The cell culture conditions were described previously [11]. Cells used for the experiments were in the logarithmic phase of growth.
Apoptosis induced by cisplatin and measured by flow cytometry
Cells were treated with a clinical dose (30 μM) of cisplatin for 4 h, and were further cultured in cisplatin-free medium for different time periods. The treated cells were rinsed, trypsinized, pelleted and resuspended in cold PBS at the desired time. To assay the apoptosis, the cells were fixed dropwise with ice-cold 100% ethanol to yield a final concentration of 70%. The suspended cells were stored at 4°C overnight, and then washed three times with PBS to remove the ethanol. The DNA of the cells was labeled by propidium iodide as described previously [12]. The cell cycle and the apoptotic peak were analyzed using Becton-Dickinson FACS-420 flow cytometry equipped with 2 W argon laser and tuned to 488 nm excitation and 525 nm emission wavelength.
1H-NMR measurements
The cells for 1H-NMR samples cultured as described above were harvested and resuspended in cold 1 × PBS. Afterwards, the cells were washed twice with PBS and subsequently with PBS-D2O to diminish NMR signal interference by H2O. The single cells were resuspended in PBS-D2O (1 × 107 cells/mL) for 1H-NMR experiments. All the 1H-NMR data were collected on Bruker Avance DMX600 spectrometer. Samples were spun at the speed of 17 Hz to avoid cell sedimentation during data collection at 37°C. 128 scans were recorded for each FID. The interference from residual water was eliminated using the WATERGATE technique [34]. The chemical shifts of the resonance groups were referenced to internal standard DDS (2,2-Dimethyl-2-silapentane-5-sulfonic acid 20 μM) at 0 ppm. Data processing and integration of the peak area of the resonances were performed with Felix 98.0 software. The resonances of chemical groups monitored by 1H-NMR were as following [13]: Methyl (CH3) resonances from fatty acids integrated between 0.75 and 1.10 ppm; menthylene (CH2) resonances from fatty acids between 1.15 and 1.5 ppm; Glu between 2.5 and 1.8 ppm; Ct between 2.9 and 3.1 ppm; N-trimethyl of Chol between 3.3 and 3.1 ppm; Ino between 3.6 and 3.5 ppm; and Eth between 3.8 and 3.7 ppm. The lipid resonance areas were reported as the ratios to CH3, and the metabolite areas as the ratios to Ct [13].
31P-NMR measurements
31P-NMR spectra of the intact cells were obtained on a Bruker Avance DPX400 spectrometer operating at 161.943 MHz phosphorus frequencies at 22°C. For each 31P-NMR experiment, the treated cells (5 × 108 cells/mL) were rinsed, trypsinized, pelleted and resuspended in cold isotonic mixture of RPMI 1640, supplemented by 40 mM glucose, 20 mM HEPES (pH 7.4) to provide sufficient sustenance and buffering capacity, and 40% (V/V) percoll to prevent cells sedimentation during 31P-NMR experiments. 0.6 mL cell suspension was transferred to a 5 mm NMR tube, which was then quickly placed in the magnet. The data were recorded with a spectral width of 50 ppm and 8 K data point, 2000 scans were collected for each FID. The 31P signals were referenced to an external standard of 85% phosphoric acid (2.3 ppm) and for the quantitative analysis; the peak areas were reported as the ratios to methylene-diphosphonate [14].
Microviscosity of plasma membrane measured using TMA-DPH
2 mM of stock solution of TMA-DPH dissolved in tetrahydrofuran was stored in the dark at 4°C. Cells suspended in PBS (1 × 106cells/mL) were incubated in a final concentration of 2 μM TMA-DPH for 20 min at 37°C. Experimental methods were according to Pangdey et al. [35]. The fluorescence anisotropy, γ, was obtained from the relation γ = 2P/(3-P) where P is polarization. The microviscosity of the plasma membrane was calculated by the Perrin equation γ0/γ = [1 + C(γ)·T·τ]/Η, where γ0 = limiting fluorescence anisotropy (0.36 for DPH), C(γ) = A parameter relating to the molecular shape and location of the transition dipoles of rotating fluorophore. T = absolute temperature, τ = excited state life lime (for DPH 11.4 ns), Η = microviscosity of the medium. The variation of C (γ) or τ for a system labeled with same probe and temperature is considerable smaller than the variation of Η. Therefore, the quantity (γ0/γ-1)-1 is taken as the apparent microviscosity, 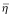 , of the medium. C (γ)·T·τ = 2.4 poise. The apparent microviscosity (
, of the medium. C (γ)·T·τ = 2.4 poise. The apparent microviscosity (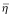 ) = 2.4γ/(0.362-γ) poise.
) = 2.4γ/(0.362-γ) poise.
Abbreviation
MDR, multidrug resistance; NMR, nuclear magnetic resonance; Glu, glutamine-glutamate; Ct, creatine-creatinine; Chol, choline and derivative compounds. Ino, inositol; Eth, ethanolamine; TMA-DPH, 6-diphenyl-1, 3, 5-hexatrine; PME, phosphomonoesters; FID, free induction decay; DDS, 2, 2-Dimethyl-2-silapentane-5-sulfonic acid.
Authors' contributions
Zhenhua Huang conceived of the study, carried out the cell biology studies and drafted the manuscript. Yufeng Tong carried out NMR experiments. Jinfeng Wang participated in its design and coordination. Youguo Huang also participated in the design of the study and revised the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgments
This work was generously supported by Key Project 39730130 from the National Nature Science Foundation of China and Project KJ951-B1-609 from the Chinese Academy of Science. The authors appreciate Prof Adi Aszalos for his critical reading of the manuscript of this paper. The authors are grateful to Prof. Shu-yi Liu who kindly provided the cell lines and Ms. Shu-hua Zhou for helping in setting up 31P-NMR experiments.
Contributor Information
Zhenhua Huang, Email: hzh2008@btamail.net.cn.
Yufeng Tong, Email: tong@nlbnmr.ibp.ac.cn.
Jinfeng Wang, Email: jfw@sun5.ibp.ac.cn.
Youguo Huang, Email: huang@sun5.ibp.ac.cn.
References
- Lehnert M. Multidrug resistance in human cancer. J Neurooncol. 1994;22:239–43. doi: 10.1007/BF01052927. [DOI] [PubMed] [Google Scholar]
- Zgurskaya HI, Nikaido H. Multidrug resistance mechanisms: drug efflux across two membranes. Mol Microbiol. 2000;37:219–25. doi: 10.1046/j.1365-2958.2000.01926.x. [DOI] [PubMed] [Google Scholar]
- Cleary I, Doherty G, Moran E, Clynes M. The multidrug-resistant human lung tumor cell line DLKP-A 10 expression novel drug accumulation and a sequestration system. Biochem Pharmacol. 1997;53:1493–502. doi: 10.1016/S0006-2952(97)00003-8. [DOI] [PubMed] [Google Scholar]
- Clynes M, Daly C, NicAmhlaoibh R, Cronin D, Elliott C, O'Connor R, O'Doherty T, Connolly L, Howlett A, Scanlon K. Recent developments in drug resistance and apoptosis research. Crit Rev Oncol Hematol. 1998;28:181–205. doi: 10.1016/S1040-8428(98)00018-3. [DOI] [PubMed] [Google Scholar]
- Grunicke H, Hofman J. Cytotoxic and cytostatic effects of antitumor agents induced at the plasma membrane level. Pharmacol Ther. 1992;55:1–30. doi: 10.1016/0163-7258(92)90027-W. [DOI] [PubMed] [Google Scholar]
- Koert NJ, Burger Rutger WHM Staffhorst, Ben De Kruijiff. Interaction of the anti-cancer drug cisplatin with phosphatidylserine in intact and semi-intact cells. Biochica et Biophysica Acta. 1999;1419:43–54. doi: 10.1016/s0005-2736(99)00052-8. [DOI] [PubMed] [Google Scholar]
- Escriba PV, Ferrer_Montiel AV, Ferragut JA, Gonzalez_Ros JM. Role of membrane lipids in the interaction of daunomycin with plasma membranes from tumor cells: implications in drug-resistance phenomena. Biochemistry. 1990;29:7275–7282. doi: 10.1021/bi00483a017. [DOI] [PubMed] [Google Scholar]
- May GL, Wright LC, Dye M, Mackinnon WB, Fox RM, Mountford CE. Plasma lipid composition of vinblastine sensitive and resistant human leukemic lymphoblasts. Int J Cancer. 1988;42:728–733. doi: 10.1002/ijc.2910420517. [DOI] [PubMed] [Google Scholar]
- Litman T, Nielsen D, Skovsgaard T, Bukhave K. Lipid composition of sensitive and multidrug resistant ascites tumor cells. Cell Pharmacol. 1995;2:9–14. [Google Scholar]
- Ferretti A, Knijn A, Iorio E, Pulciani S, Giambenedetti M, Molinari A, Meschini S, Stringaro A, Calcabrini A, Freitas I, Strom R, Arancia G. Podo: Biophysical and structural characterization of 1H-NMR-detectable mobile lipid domains in NIH-3T3 fibroblasts. Biochim Biophys Acta. 1999;1438:329–348. doi: 10.1016/S1388-1981(99)00071-2. [DOI] [PubMed] [Google Scholar]
- Liang Xingjie, Huang Youguo. Alteration of membrane lipid biophysical properties and resistance of human lung adenocarcinoma A549 cells to cisplatin. Sciences in China (Series C) 2001;44:25–32. doi: 10.1007/BF02882069. [DOI] [PubMed] [Google Scholar]
- Patricia JudsonL, Joanna WatsonM, Paola GehrigA, Weasley FowlerC, Jr, Stephen H. Cisplatin inhibits paclitaxel-induced apoptosis in cisplatin-resistant ovarian cancer cell lines: possible explanation for failure of combination therapy. Cancer Research. 1999;59:2425–2432. [PubMed] [Google Scholar]
- Laurence Le Moyec, Roger Tatoud, Armelle Degeorges, Cynthia Calabresse, Guy Bauza, Michel Eugene, Fabien Calvo. Proton nuclear magnetic resonance spectroscopy reveals cellular lipids involved in resistance to adriamycin and taxol by the K562 leukemia cell line. Cancer Research. 1996;56:3461–3467. [PubMed] [Google Scholar]
- Foluso Adebodun, Jan PostFM. 31P NMR characterization of cellular metabolism during dexamethasone induced apoptosis in human leukemic cell lines. Journal of Cellular Physiology. 1994;158:180–186. doi: 10.1002/jcp.1041580122. [DOI] [PubMed] [Google Scholar]
- Ferretti G, Zolese G, Curatola G, Jezequel AM, Benedetti Membrance heterogeneity in isolated rat hepatocytes and liver plasma membrane subfraction: a comparative study using DPH and its cationic derivative TMA-DPH. Biochimica et Biophysica Acta. 1993;1147:245–250. doi: 10.1016/0005-2736(93)90009-O. [DOI] [PubMed] [Google Scholar]
- Mountford CE, Mackinnon EB. Proton magnetic resonance spectroscopy of lymphocytes: an historical perspective. Immunmethods. 1994;4:98–112. doi: 10.1006/immu.1994.1012. [DOI] [PubMed] [Google Scholar]
- Singh JK, Dasgupta A, Adayev T, Shahmehdi SA, Hammond D, Banerjee P. Apoptosis is associated with an increase in saturated fatty acid containing phospholipids in the neuronal cell line, HN2-5. Biochim Biophys Acta. 1996;1304:171–178. doi: 10.1016/S0005-2760(96)00134-8. [DOI] [PubMed] [Google Scholar]
- Mountford CE, Grossman G, Reid G, For RM. Characterization of transformed cells and tumor by proton nuclear magnetic resonance spectroscopy. Cancer Research. 1982;42:2270–2277. [PubMed] [Google Scholar]
- May GL, Wright LC, Holmes KT, Williams PG, Smith ICP, Wright PE, Fox RM, Mountford CE. Assignment of methylene proton resonances in NMR spectra of embryonic and transformed cells to plasma membrane triglyceride. J Biochem. 1986;261:3048–3054. [PubMed] [Google Scholar]
- Michal Neeman, Hadassa Degani. Metabolic studies of estrogen and tamoxifen-treated human cancer cells by nuclear magnetic resonance spectroscopy. Cancer Research. 1989;49:589–594. [PubMed] [Google Scholar]
- Liora Bogin, Moshe PapaZ, Sylvie Polak-Charcon, Hadassa Degani. TNF-induced modulations of phosphlipid metabolism in human breast cancer cells. Biochimica et Biophysica Acta. 1998;1392:217–232. doi: 10.1016/s0005-2760(98)00035-6. [DOI] [PubMed] [Google Scholar]
- Cuadrado A, Carnero A, Dolfi F, Jimenez B, Lacal JC. Phosphorylcholine: a novel second messenger for mitogenic activity of growth factors. Oncogene. 1993;8:2959–2968. [PubMed] [Google Scholar]
- Dedon PC, Borch RF. Characterization of the reactions of platinum antitumor agents with biologic and nonbiologic sulfur-containing nucleophiles. Biochem Pharmacol. 1987;36:1955–1964. doi: 10.1016/0006-2952(87)90494-1. [DOI] [PubMed] [Google Scholar]
- Mistry P, Loh SY, Kelland LR. EVect of buthionine sulfoximine on PtII and PtIV drug accumulation and the formation of glutathione conjugates in human ovarian-carcinoma cell lines. Int J Cancer. 1993;55:849–856. doi: 10.1002/ijc.2910550526. [DOI] [PubMed] [Google Scholar]
- Eastman A. Cross-linking of glutathione to DNA by cancer chemotherapeutic platinum coordination complexes. Chem Biol Interact. 1987;61:241–248. doi: 10.1016/0009-2797(87)90004-4. [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Ali-Oaman F. Glutathione-associated cis-diammine-dichloroplatinum (II) metabolites and ATP-dependent efflux from leukemia cells. Journal of Biological Chemistry. 1993;268:20116. [PubMed] [Google Scholar]
- Andrews PA, Murphy MP. Howell SB. DiVerential potentiation of alkylating and platinating agent cytotoxicity in human ovarian-carcinoma cells by glutathione depletion. Cancer Res. 1985;45:6250–6253. [PubMed] [Google Scholar]
- Teicher BA, Holden SA, Kelley MJ. Characterization of a human squamous-carcinoma cell line resistant to cisp-diamminedichloroplatinum (II) Cancer Res. 1987;47:388–393. [PubMed] [Google Scholar]
- Perez RP. Cellular and molecular determinants of cisplatin resistance. European Journal of Cancer. 1998;34:1535–1542. doi: 10.1016/S0959-8049(98)00227-5. [DOI] [PubMed] [Google Scholar]
- Ahn H, Lee E, Kim K, Lee C. EVect of glutathione and its related enzymes on chemosensitivity of renal cell carcinoma and bladder carcinoma cell lines. J Urol. 1994;151:263–267. doi: 10.1016/s0022-5347(17)34929-7. [DOI] [PubMed] [Google Scholar]
- Behrens BC, Hamilton TC, Masuda H. Characterization of a cis-diammine-dichloroplatinum (II)-resistant human ovarian cancer cell line and its use in evaluation of platinum analogues. Cancer Res. 1987;47:414–418. [PubMed] [Google Scholar]
- Mistry P, Kelland LR, Abel G, Sidhar S, Harrap KR. The relationships between glutathione, glutathione-S-transferase and cytotoxicity of platinum drugs and melphalan in eight human ovarian cancer cell lines. Br J Cancer. 1991;64:215–220. doi: 10.1038/bjc.1991.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Ruff C, Malfoy B, Scholl S, Zafrani B, Dutrillaux B. CSTp gene is frequently coamplified with INT2 and HSTF1 proto-oncogenes in human breast cancers. Oncogene. 1991;6:403–406. [PubMed] [Google Scholar]
- Piotto M, Saudek V, Sklenar V. Gradient tailored excitation for single-quantum NMR-spectroscopy of aqueous-solutions. J Biomol NMR. 1992;2:661–665. doi: 10.1007/BF02192855. [DOI] [PubMed] [Google Scholar]
- Pangdey BN, Mishra KP. Radiation induced oxidative damage modification by cholesterol in liposomal membrance. Radiation Physics and Chemistry. 1999;54:481–489. doi: 10.1016/S0969-806X(98)00297-7. [DOI] [Google Scholar]


