Abstract
Background
Recent observations indicate that human tumorous breast tissue metabolizes progesterone differently than nontumorous breast tissue. Specifically, 5α-reduced metabolites (5α-pregnanes, shown to stimulate cell proliferation and detachment) are produced at a significantly higher rate in tumorous tissue, indicating increased 5α-reductase (5αR) activity. Conversely, the activities of 3α-hydroxysteroid oxidoreductase (3α-HSO) and 20α-HSO enzymes appeared to be higher in normal tissues. The elevated conversion to 5α-pregnanes occurred regardless of estrogen (ER) or progesterone (PR) receptor levels. To gain insight into these differences, the activities and expression of these progesterone converting enzymes were investigated in a nontumorigenic cell line, MCF-10A (ER- and PR-negative), and the three tumorigenic cell lines, MDA-MB-231 (ER- and PR-negative), MCF-7 and T-47D (ER- and PR-positive).
Methods
For the enzyme activity studies, either whole cells were incubated with [14C]progesterone for 2, 4, 8, and 24 hours, or the microsomal/cytosolic fraction was incubated for 15–60 minutes with [3H]progesterone, and the metabolites were identified and quantified. Semi-quantitative RT-PCR was employed to determine the relative levels of expression of 5αR type1 (SRD5A1), 5αR type 2 (SRD5A2), 20α-HSO (AKR1C1), 3α-HSO type 2 (AKR1C3), 3α-HSO type 3 (AKR1C2) and 3β-HSO (HSD3B1/HSD3B2) in the four cell lines using 18S rRNA as an internal control.
Results
The relative 5α-reductase activity, when considered as a ratio of 5α-pregnanes/4-pregnenes, was 4.21 (± 0.49) for MCF-7 cells, 6.24 (± 1.14) for MDA-MB-231 cells, 4.62 (± 0.43) for T-47D cells and 0.65 (± 0.07) for MCF-10A cells, constituting approximately 6.5-fold, 9.6-fold and 7.1 fold higher conversion to 5α-pregnanes in the tumorigenic cells, respectively, than in the nontumorigenic MCF-10A cells. Conversely, the 20α-HSO and 3α-HSO activities were significantly higher (p < 0.001) in MCF-10A cells than in the other three cell types. In the MCF-10A cells, 20α-HSO activity was 8-14-fold higher and the 3α-HSO activity was 2.5-5.4-fold higher than in the other three cell types. The values of 5αR:20α-HSO ratios were 16.9 – 32.6-fold greater and the 5αR:3α-HSO ratios were 5.2 – 10.5-fold greater in MCF-7, MDA-MB-231 and T-47D cells than in MCF-10A cells. RT-PCR showed significantly higher expression of 5αR1 (p < 0.001), and lower expression of 20α-HSO (p < 0.001), 3α-HSO2 (p < 0.001), 3α-HSO3 (p < 0.001) in MCF-7, MDA-MB-231 and T-47D cells than in MCF-10A cells.
Conclusion
The findings provide the first evidence that the 5αR activity (leading to the conversion of progesterone to the cancer promoting 5α-pregnanes) is significantly higher in the tumorigenic MCF-7, MDA-MB-231 and T-47D breast cell lines than in the nontumorigenic MCF-10A cell line. The higher 5αR activity coincides with significantly greater expression of 5αR1. On the other hand, the activities of 20α-HSO and 3α-HSO are higher in the MCF-10A cells than in MCF-7, MDA-MB-231 and T-47D cells; these differences in activity correlate with significantly higher expression of 20α-HSO, 3α-HSO2 and 3α-HSO3 in MCF-10A cells. Changes in progesterone metabolizing enzyme expression (resulting in enzyme activity changes) may be responsible for stimulating breast cancer by increased production of tumor-promoting 5α-pregnanes and decreased production of anti-cancer 20α – and 3α-4-pregnenes.
Background
Previous studies [1] have shown that breast tissue converts progesterone to two classes of 21-carbon steroids: those that retain the C4–5 double bond (4-pregnenes) and those that are 5α-reduced (5α-pregnanes). The conversion to 5α-pregnanes, requiring the action of 5α-reductase (5αR), is significantly higher in tumorous than in nontumorous human breast tissue [1]. Exposure of human breast cell lines (MCF-7, MCF-10A, and ZR-75-1) to 5α-pregnanes results in changes associated with neoplasia, including increased proliferation and decreased attachment [1], depolymerization of F-actin [2] and decreases in adhesion plaque-associated vinculin [2]. Exposure to 4-pregnenes results, in general, in opposite (anti-cancer-like) effects [1,2]. Breast tissue has also been shown to exhibit 3α-hydroxysteroid oxidoreductase (3α-HSO), 20α-HSO and 3β-HSO activities [1]. Activities of 3α-HSO and 20α-HSO were higher in the normal tissue of matched tissue samples [1]. Additionally, specific but separate, high-affinity receptors for the 5α-pregnane, 5α-pregnane-3,20-dione and the 4-pregnene, 3α-hyroxy-4-pregnen-20-one, have been identified in the plasma membrane fractions of breast cancer cells [3].
The results from the previous studies [1-3] imply important cancer regulating functions for autocrine/paracrine progesterone metabolites and suggest regulatory roles for 5αR, 20α – and 3α(β)-HSO activities in human breast cancer progression. In order to further study the role of these enzymes in breast cancer it was of interest to determine if breast cell lines exhibit these activities and if gene expression levels can be correlated to the levels of activity. For comparisons we chose a breast epithelial cell line (MCF-10A) known to be nontumorigenic [4] and three human breast cancer cell lines (MCF-7, MDA-MB-231 and T-47D) known to be tumorigenic [5,6] in athymic nude mice. The MCF-10A cell line was derived from human fibrocystic mammary tissue and is estrogen (ER-) and progesterone (PR-) receptor negative [4]. The MCF-7, MDA-MB-231 and T-47D cell lines were derived from human breast adenocarcinoma, MCF-7 and T-47D cells being ER+ and PR+ [7] and estrogen-dependent for tumorigenicity [5,6], and MDA-MB-231 cells being ER- and PR- [8] and will form tumors with or without supplemental estrogen [5]. Therefore, the objectives of the current studies were (a) to compare the activities of 5αR, 20α-HSO, 3α-HSO and 3β-HSO in one nontumorigenic and three tumorigenic human breast cell lines, and (b) to determine if differences in level of enzyme activities might be related to differences in specific mRNA expression.
These studies show that 5αR activity is significantly higher in MCF-7, MDA-MB-231 and T-47D cells than in MCF-10A cells, resulting in significantly higher 5α-pregnane:4-pregnene ratios in the tumorigenic (MCF-7, MDA-MB-231 and T-47D) cell lines. Conversely, 20α-HSO and 3α-HSO activities are significantly higher in the MCF-10A cell line. The results also provide the first demonstration of the expression of messenger RNA for 5αR type 1 (5αR1; SRD5A1), 5αR type 2 (5αR2; SRD5A2), 20α-HSO (AKR1C1), 3α-HSO type 2 (3α-HSO2; AKR1C3), 3α-HSO type 3 (3α-HSO3; AKR1C2) and 3β-HSO in breast cell lines. Moreover, they show opposing expression patterns for 5αR1 and 20α-(3α-)-HSOs in nontumorous (MCF-10A) compared to the three tumorigenic cell lines.
Methods
Chemicals
[4-14C]Progesterone (55.4 mCi/mmol) and [1,2,6,7-3H]progesterone (114.8 Ci/mmol) were purchased from Perkin Elmer Life Sciences (New England Nuclear) (Woodbridge, Ontario). [1,2,6,7-3H]3α-Hydroxy-4-pregnen-20-one (3αHP) was synthesized from [1,2,6,7-3H]progesterone by published procedures [9]. Unlabeled 3αHP, 3βHP, and 4-pregnene-3α (β),20α(β)-diols were prepared as described previously [10,11]. Other unlabeled steroids were purchased from Steraloids Inc (Wilton, N.H.) or Sigma Chemical Co. (Oakville, ON). Cell culture media, insulin, penicillin, streptomycin, hydrocortisone, cholera toxin, and epidermal growth factor were from Sigma and serum was purchased from Invitrogen (Burlington, ON). N-methyl-bis-trifluoroacetamide and N,O-bis(trimethylsilyl)trifluoroacetamide were obtained from Pierce Chemical Co. (Rockford, IL). Other chemicals and solvents were of appropriate analytical grade and were purchased from BDH Inc., (Toronto, ON), VWR (Mississauga, ON) or Fisher Scientific Ltd. (Toronto, ON). Thin layer chromatography (TLC) plates (EM Science, silica gel 60 F254, 250 μm thickness (20 × 20 cm; 2.5 × 7.5 cm) were obtained from VWR. Medical X-ray film (X-OMAT R) was from Kodak.
Cell Culture
MCF-7 cells came from Dr. R. Shiu through the courtesy of Dr. J. Wimalasena and the MDA-MB-231, T-47D and MCF-10A cells were purchased from the American Type Culture Collection (Manassas, VA). The MCF-7, MDA-MB-231 and MCF-10A cells were grown in a 1:1 Ham's F12 Medium and Dulbecco's Modified Eagle's Medium with 2 mM L-glutamine. The T-47D cells were grown in RPMI-1640 with L-glutamine. The medium was supplemented with 5% calf serum (MCF-7), 10% calf serum (MDA-MB-231), 10% Fetal Bovine Serum (T-47D) or 5% horse serum (MCF-10A), 100 units/ml penicillin, 78 units/ml streptomycin, 10 μg/ml insulin and 1.2 mg/ml sodium bicarbonate. During the subculture period, MCF-10A cells were further supplemented with 0.5 mg/L hydrocortisone, 0.1 μg /ml cholera toxin and 0.02 μg /ml epidermal growth factor as recommended [4]. Cells were grown in T75 flasks until 80% confluence before harvesting (for cell-free cytosolic/microsomal enzyme activity studies) or subculturing in 35 mm dishes (for expression or whole cell enzyme activity studies). For the expression studies, cells were seeded at 300,000 cells per 35 mm dish in their own media, allowed to attach for 24 hours, and then grown in serum free MCF-7 media for 48 hours prior to harvest. For the in vivo enzyme activity studies, cells were seeded at 106 cells, allowed to attach for 24 hours, medium was replaced with medium containing [14C]progesterone and incubation was continued for 2, 4, 8, or 24 hours before termination and extraction as reported [1,12,13]. Cells were maintained in a humidified incubator at 37°C and a 5% CO2 atmosphere.
Enzyme Activities – Whole Cells in Culture
For the in vivo enzyme activity studies cells were seeded in 35 mm plastic (Falcon) dishes at approximately 750 K cells per dish and allowed to attach overnight. Some dishes were used to determine cell numbers by hemocytometer at 0 and 24 hours after the start of incubations. Medium was removed and replaced with fresh medium containing [14C]progesterone (0.2 μCi). Cell numbers were determined by hemocytometer in parallel dishes incubated without [14C]progesterone for 0 or 24 hours. Additional controls consisted of incubations of medium containing [14C]progesterone in the absence of cells. Each treatment consisted of 3–4 dishes and the progesterone metabolism of each cell type was examined in at least two separate experiments. Incubations were terminated at 0 (control), 2, 4, 8 and 24 hours by removing cells and media to siliconized glass extraction tubes on ice and adding 2.0 ml ice cold 0.05 M NaOH and 6.0 ml ether:chloroform (4:1). Known amounts of unlabeled steroid standards were added for recovery calculations and identification purposes.
The [14C]progesterone metabolites were extracted three times with the ether:chloroform mixture, the extracts were delipidated, run two-dimensionally on TLC, identified by TLC-autoradiograpy, high performance liquid chromatography (HPLC), derivatization, capillary gas chromatography-mass spectrometry and quantified by scintillation spectrometry as previously described [1,12,13]. The amount of each metabolite produced was determined as percent of the total, and, following corrections for procedural losses, was calculated as ng per hour per 106 cells. Because these values had begun to decrease somewhat for the main metabolites at 24 hours, the values given in this report are those determined from the 4- and 8-hour incubations.
Enzyme Activities in Microsomal/Cytosolic Fractions
Each cell type, grown in 8–10 T75 flasks until 80–90% confluent, was harvested, washed in balanced salt solution, and brought up in 3 mL of ice-cold phosphate buffered sucrose/glycerol solution (Na2HPO4, 0.8 mM; NaH2PO4, 0.2 mM; sucrose 250 mM, EDTA, 1.0 mM; glycerol, 20% v/v; pH 7.2). Cells were homogenized in a tight-fitting glass-glass homogenizer (40 strokes), centrifuged (0°C) at 12,000 × g for 10 minutes, and the supernatants (designated as the microsomal/cytosolic fractions) were stored at -80°C until used. Protein concentrations were determined by the method of Bradford [14].
Enzyme Assays. Reductive enzyme assays were conducted by incubating cytosolic/microsomal fractions (100 μL) in phosphate buffer (Na/Na, 0.05 M, pH 7.0) containing NADH and NADPH (0.4 mM final concentration) and [1,2,6,7 3H]progesterone (350,000 dpm per 455 pg) for 15, 30, or 60 minutes in a shaking water bath at 37°C. The reaction was stopped by immersing the tubes in ice, adding 0.5 mL ice-cold 0.05 M NaOH and 3.0 mL ether/CH2Cl2 (9:1). Following two extractions (3 mL each) with ether/CH2Cl2, the steroid metabolites were separated by TLC (25 × 75 mm; chloroform/acetone, 9:1) and HPLC (C18, methanol/water, 72:28) and quantified by scintillation spectrometry.
RNA Isolation and Reverse Transcription
Total RNA was extracted from the cells using TRIzol® reagent (Invitrogen) following the manufacturer's protocol. RNA yields were measured by spectrophotometry and RNA integrity was analysed by agarose gel electrophoresis. Prior to reverse transcription, RNA was treated with DNase I for 30 minutes at 37°C using a DNA-free™ kit (Ambion). Complementary DNA (cDNA) was obtained from 2.0 μg of DNase treated total RNA using 200 U Moloney Murine Leukemia Virus (MMLV) reverse transcriptase (Invitrogen) and random hexamer primers (Invitrogen) in a total volume of 20 μl following the manufacturers protocol. To exclude amplification of genomic DNA, experiments included conditions in which the reverse transcriptase enzyme was omitted.
Semi-Quantitative RT-PCR
Prior to semi-quantitative RT-PCR, primers for the following commonly used housekeeping genes were tested for variability of expression between cell lines: 18S rRNA, β-actin, glyceraldehyde-3-phosphate dehydrogenase (G3PDH) and cyclophilin. Based on least variation of expression between cell lines, 18S was chosen as the control for the enzyme expression studies. The PCR primers were purchased from Invitrogen with sequences given in table 1[15-17,43]. PCR conditions for the primer sets were as follows: 95°C denature for 4 minutes followed by cycling, each cycle with a 20 second denature at 94°C, a 30 second anneal at 62°C, and a 30 second extension at 72°C. Cycling was followed by a final extension for 4 minutes at 72°C. For each reaction an 2 μl aliquot of cDNA product was amplified in a 25 μl total volume using 1.25 U of Platinum Taq DNA Polymerase (Invitrogen), 2 mM MgCl, 0.2 mM dNTP's, 2 mM each primer. The same cycling conditions and PCR reagent concentrations were used for each set of primers. The kinetics of the PCR reactions for each primer set was determined by varying the amount of template and the number of cycles. All PCR product identities were confirmed by separation on a 1.5% agarose gel and sequencing at the Robarts Research Institute Sequencing Facility (London, ON).
Table 1.
Primer sequences (upstream; downstream) used in the RT-PCR analyses.
| cDNA | Sequence | Size | Reference |
| SRD5A1 (5αR1) | 5'-TGG CGC TTC TCT ATG GAC TT-3' 5'-GGA AGC AAC ACT GCA GTT GA-3' | 368 bp | [15] |
| SRD5A2 (5αR2) | 5'-CAT ACG GTT TAG CTT GGG TGT-3' 5'-GCT TTC CGA GAT TTG GGG TAG-3' | 315 bp | [16] |
| AKR1C1 (20α(3α)HSD) | 5'-GTA AAG CTT TAG AGG CCA C-3' 5'-CAC CCA TGC TTC TTC TCG G-3' | 590 bp | [43] |
| AKR1C2 (3αHSD3) | 5'-GTA AAG CTC TAG AGG CCG T-3' 5'-CAC CCA TGG TTC TTC TCG A-3' | 590 bp | [43] |
| AKR1C3 (3α(17β)HSD2) | 5'-GTA AAG CTT TGG AGG TCA C-3' 5'-CAC CCA TCG TTT GTC TCG T-3' | 590 bp | [43] |
| AKR1C4 (3αHSD1) | 5'-ACA GAG CTG TAG AGG TCA C-3' 5-CAC CCA TAG TTT ATG TCG T-3' | 590 bp | [43] |
| HSD3B1 &HSD3B2 (3βHSD) | 5'-GCT GGA GCT GCC TTG TGA C-3' 5'-CCC GGC TAC CTC TAT GCT ACT-3' | 383 bp | (M.J. Lewis) |
| 18S rRNA | 5'-GTA ACC CGT TGA ACC CCA TT-3' 5'-CCA TCC AAT CGG TAG TAG CG-3' | 131 bp | [17] |
For each cDNA sample, aliquots were amplified for 13 cycles with the 18S rRNA primers, 25 cycles with the 5αR1 primers, 33 cycles with the 5αR2 primers and 22 and 25 cycles with the 20α-HSD, 3α-HSD2 and 3α-HSD3 primers respectively. Products were separated on 9% polyacrylamide gels and the intensities of the appropriate bands were quantified by Quantity One 4.2.1 Gel Doc Software (BioRad Laboratories) and expressed as total pixel value, based on intensity and number of pixels per band. Results are expressed as ratios of the total pixel value of the band of interest to the total pixel value of the 18S rRNA band. Each ratio represents the average of duplicate PCRs performed for each gene from 5–7 replicate experiments.
Statistics
Results are expressed as mean ± SEM and were analysed by one-way ANOVA followed by Tukey's pairwise comparisons to detect significant differences between groups, p < 0.05, using the computer program InStat (GraphPad, San Diego, CA).
Results
Enzyme Activities in Cultured Cells
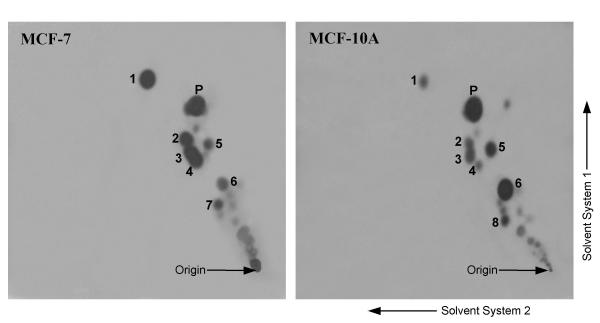
Figure 1 shows sample autoradiographs of TLC-separated [14C]progesterone and its metabolites formed by two of the four (MCF-7 and MCF-10A) cell lines during an 8 h incubation. The main metabolites of [14C]progesterone were identified as: 5α-pregnane-3,20-dione, 5α-pregnan-3α-ol-20-one, 5α-pregnan-20α-ol-3-one, 5α-pregnan-3β-ol-20-one, 5α-pregnane-3α (β), 20α-diols, 4-pregnen-3α-ol-20-one (3αHP), 4-pregnen-20α-ol-3-one (20αDHP), and 4-pregnene-3α, 20α-diol (The structures of the metabolites are shown in Figure 6). The 5α-reductase activity was calculated from the total amounts of all 5α-reduced metabolites. The 3α-HSO, 3β-HSO, and 20α-HSO activities were calculated from the total amounts of all 3α-hydroxy, 3β-hydroxy, and 20α-hydroxy metabolites, respectively.
Figure 1.
Sample autoradiographs of TLCs showing 14C-labeled metabolites of progesterone in MCF-7 and MCF-10A cells following an 8-hour incubation with [14C]progesterone (P). The main metabolites identified and quantified for these enzyme activity studies are: (1) 5α-pregnane-3,20-dione, (2) 5α-pregnan-3α-ol-20-one, (3) 5α-pregnan-20α-ol-3-one, (4) 5α-pregnan-3β-ol-20-one, (5) 4-pregnen-3α-ol-20-one (3αHP), (6) 4-pregnen-20α-ol-3-one (20αDHP), (7) 5α-pregnane-3α (β), 20α-diol, (8) 4-pregnene-3α,20α-diol. The extracts were spotted at the origin and the plates were run 2 × in solvent system 1 and 3 × in solvent system 2 in the directions indicated, as described under Methods, and the films were exposed for 8 days. Note greater size/intensity of 5α-pregnane spots (Nos. 1–4, 7) in MCF-7 cells, and greater size/intensity of 4-pregnene spots (No. 5 and 6) in MCF-10A cells.
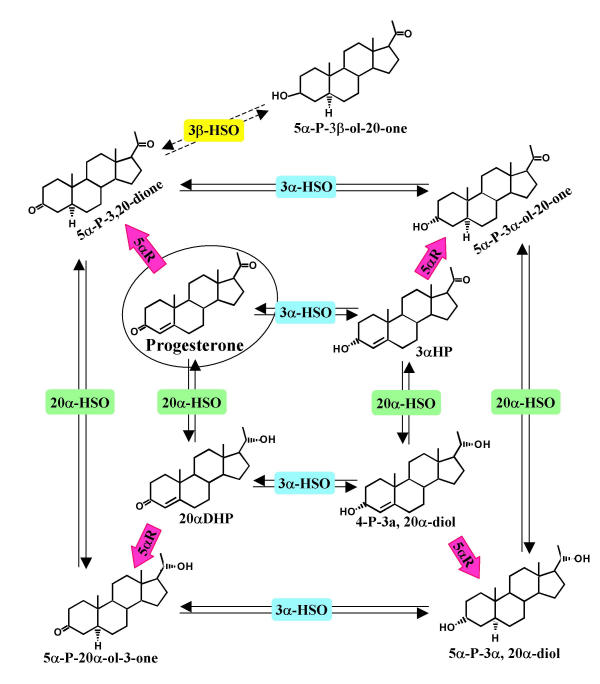
Figure 6.
Progesterone metabolizing enzymes and pathways in MCF-7, MDA-MB-231, T-47D, and MCF-10A cells. Progesterone is converted to 5α-pregnane-3,20-dione (5α-P-3,20-dione) by 5αR, to 4-pregnen-3α-ol-20-one (3αHP) by 3α-HSO, or to 4-pregnen-20α-ol-3-one (20αDHP) by 20α-HSO. Each of the 4-pregnenes can be 5α-reduced by 5αR, in a one-way reaction, to the respective 5α-pregnane. The activities of 3α-HSO and 20α-HSO are reversible and control reductive and oxidative inter-conversions within the 4-pregnenes (inner ring of metabolites) or 5α-pregnanes (outer ring of metabolites). Identification of 5α-pregnan-3β-ol-20-one as a metabolite of all four cell types, indicates the presence of 3β-HSO activity, but at this time a full pathway of 3β-reduction/oxidation is not identified.
5α-Reductase
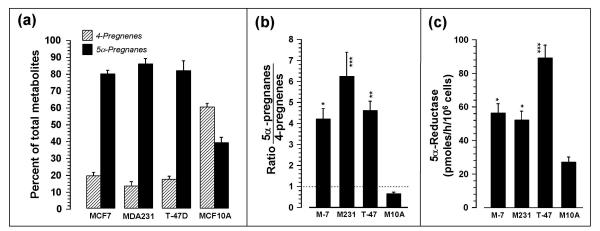
Figure 2a shows that 5α-pregnanes comprised 80–89% of all progesterone metabolites in MCF-7, MDA-MB-231 and T-47D cells, and about 39% in the MCF-10A cells. When considering the metabolites as a ratio of 5α-pregnanes/4-pregnenes (Fig. 2b) the relative values for MCF-7, MDA-MB-231 and T-47D cells are significantly (p < 0.05-p < 0.001; 6.5-, 9.6-, and 7.1-fold) greater than for MCF-10A cells. Figure 2c shows that 5αR activity, calculated as 5α-pregnanes formed (pmoles/h/106 cells), is significantly (p < 0.05-0.01) higher in MCF-7, MDA-MB-231 and T-47D cells than in MCF-10A cells.
Figure 2.
Relative 5α-reductase (5αR) activities in MCF-7, MDA-MB-231, T-47D, and MCF-10A cells, calculated (mean ± SEM; n = 3–6) as (a) percent of total metabolites, (b) ratio of 5α-pregnanes:4-pregnenes, and (c) pmoles per hour per 106 cells of 5α-reduced metabolites produced. In (a) the mean levels of 4-pregnenes are significantly different from the mean levels of 5α-pregnanes at p < 0.001. In (b) and (c), *, **, and *** indicate significant differences from MCF-10A cells at p < 0.05, p < 0.01, and p < 0.001, respectively.
20α-HSO, 3α-HSO and 3β-HSO
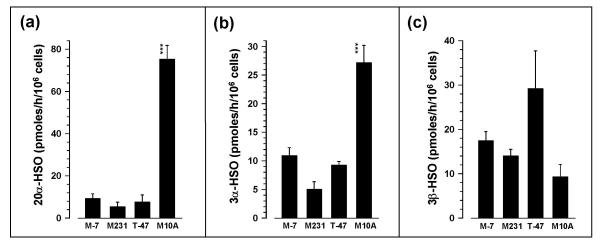
The 20α-HSO activity was not significantly different (p > 0.05) between MCF-7, MDA-MB-231 and T-47D cells but was significantly (p < 0.001; 8.09–13.98-fold) higher in MCF-10A than in the other three cell types (Fig. 3a). 3α-HSO activity was not significantly (p > 0.05) different between MCF-7, MDA-MB-231 and T-47D cells but was significantly (p < 0.001; 2.5–5.4-fold) higher in MCF-10A than in the other three cell types (Fig. 3b). 3β-HSO activities (ranging between 9.3 and 29.2 pmoles/h/106 cells) were not significantly different (p > 0.05) between cell types (Fig. 3c).
Figure 3.
Activities (mean ± SEM; n = 3–6) of (a) 20α-HSO, (b) 3α-HSO and (c) 3β-HSO in MCF-7, MDA-MB-231, T-47D, and MCF-10A cells, calculated from the metabolites as pmoles/hour/106 cells. ***, significantly different from MCF-7, MDA-MB-231, and T-47D cells at p < 0.001.
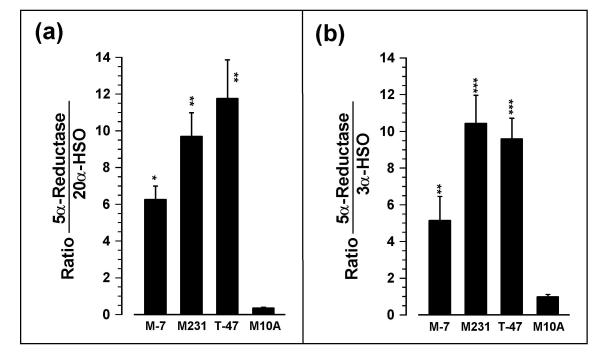
Activity Ratios
When calculated as ratio of 5αR:20α-HSO (Fig. 4a), values for MCF-7, MDA-MB-231 and T-47D cells were not significantly different (p > 0.05) from each other but were significantly (p < 0.01; 16.9-, 26.9- and 32.6-fold, respectively) greater than for MCF-10A cells. The 5αR:3α-HSO ratios for MDA-MB-231 and T-47D cells were significantly (p < 0.05) greater than for MCF-7 cells and the ratio for MCF-10A cells was significantly (p < 0.001) lower than for the other three cell lines (Fig. 4b).
Figure 4.
Ratios of (a) 5αR:20α-HSO and (b) 5αR:3α-HSO activities in MCF-7, MDA-MB-231, T-47D, and MCF-10A cells. *, **, and *** indicate significant differences from MCF-10A cells at p < 0.05, p < 0.01, and p < 0.001, respectively.
Enzyme Activities in Microsomal/Cytosolic Fractions
Incubating whole cells in their normal medium in the presence of [14C]progesterone results in both reductive and oxidative reactions promoted by the endogenous cofactors. In a cell-free system it is possible to determine more precisely the reductive or oxidative reaction velocities by supplying the corresponding cofactors and controlling the pH. More direct measurements of reductive enzyme activities were obtained by incubating microsomal/cytosolic fractions of cells with [3H]progesterone (at pH 7.0) in the presence of NADH and NADPH. The results, presented in Table 2 as the mean 5αR, 20α-HSO and 3α-HSO activities (pg/hour/mg protein or as percent of total enzyme activities) for duplicate incubations, in general are in line with the findings in cultured cells. The activity of 5αR is lowest and the activities of 20α-HSO and 3α-HSO are highest in MCF-10A cells resulting in markedly lower ratios of 5αR:20α-HSO and 5αR:3α-HSO than in MCF-7, MDA-MB-231 and T-47D cells (Table 2). The oxidative 3α-HSO activity (determined in MCF-7, T-47D and MCF-10A microsomal/cytosolic fractions using the substrate, [3H]3αHP, and cofactors, NAD and NADP, at pH 8.0) was about 8- and 18-fold higher in MCF-10A than in MCF-7 and T-47D cells, respectively (results not shown).
Table 2.
5α-Reductase, 20α-HSO and 3α-HSO activities in microsomes/cytosol of breast cell lines.
| Cells | 5αR | 20α-HSO (reductive) | 3α-HSO (reductive) | 5αR/20α-HSO | 5αR/3α-HSO |
| MCF-7 | 228.6 (86.5) | 17.9 (6.8) | 17.9 (6.8) | 12.77 | 12.77 |
| MDA231 | 186.2 (75.3) | 49.8 (20.1) | 11.3 (4.6) | 3.74 | 16.48 |
| T-47D | 82.1 (74.5) | 21.8 (19.8) | 6.3 (5.7) | 3.77 | 13.03 |
| MCF-10A | 78.3 (7.6) | 845.1 (81.4) | 114.2 (11.0) | 0.93 | 0.69 |
The 10,000 × g supernatant of cell homogenates was incubated with NADH, NADPH and [3H]progesterone, and enzyme activities were determined by quantifying metabolites formed as a result of specific enzyme actions (See Methods for details). Values (mean from two experiments) are given as pg/hour/mg protein; the values in brackets show the percent of total enzyme activities for a particular cell type.
Semi-quantitative RT-PCR
RT-PCR followed by direct product sequencing confirmed that 5αR1, 5αR2, 20α-HSO, 3α-HSO2, 3α-HSO3 and 3β-HSO type 1 are expressed in MCF-7, MDA-MB-231, T-47D and MCF-10A cells. PCR of RT reactions lacking reverse transcriptase enzyme resulted in no amplification.
To determine the range of accuracy of quantification for a specific cycle number, PCR was performed using varying amounts (1 μl, 2 μl, 4 μl, 8 μl) of MCF-7 cDNA as template. The total pixel value of respective 5αR1 (25 cycles) and 18S (13 cycles) bands, determined for 3–5 replicate amplifications, increased linearly between 1 μl and 8 μl of RT template. Linear regression analysis indicated a goodness of fit index of 0.859 and 0.967 for 5αR1 and 18S, respectively, and correlation (data vs. model) of 0.999 for each. When amplifying templates of 16.0 μl equivalent or greater the slopes decreased indicating that the software underestimated the pixel values when too much template is amplified. When less than 1.0 μl equivalent amounts of template were amplified, the bands were invisible or barely visible and the software tended to underestimate the pixel values. The results showed that quantification by this procedure is linear over an 8-fold range for any single cycle number. Based on these findings we determined that 18S could be quantified in the 4 cell types by amplifying for 13 cycles, 5αR1 could be quantified by amplifying for 25 cycles, and 5αR2 could be quantified by amplifying for 33 cycles. Due to a greater variability of expression between cell types, 20α-HSD, 3α-HSD2 and 3α-HSD3 were amplified for 22 and 25 cycles to cover a greater range of expression.
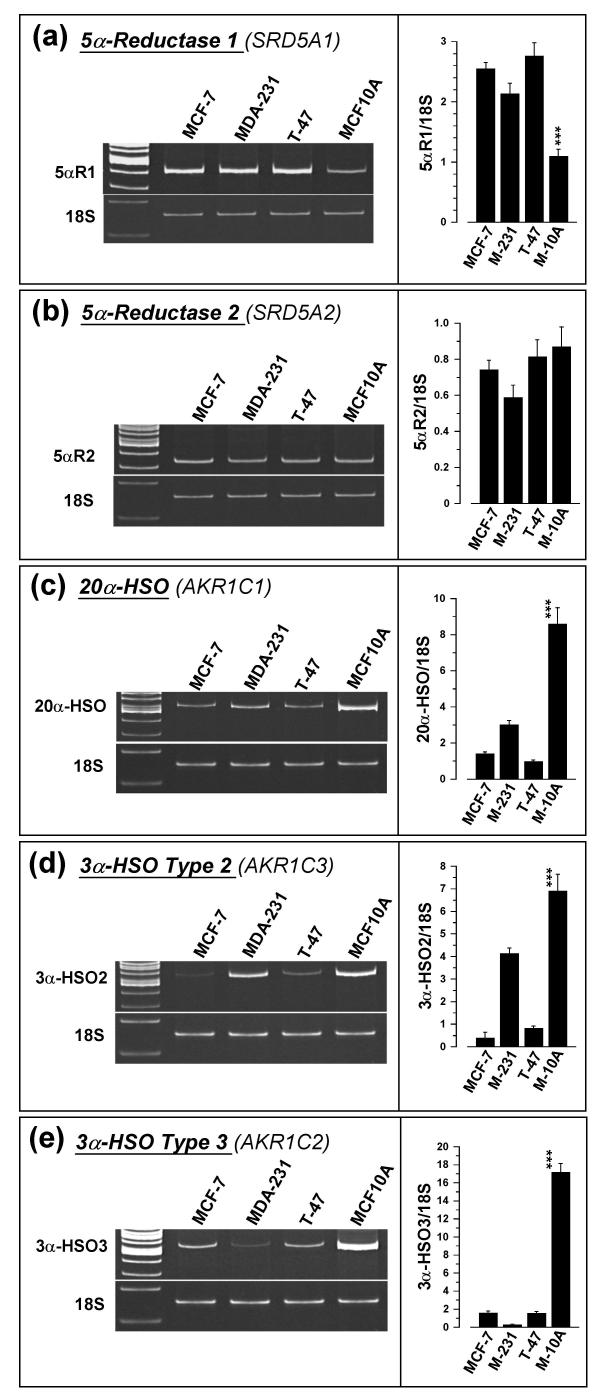
Expression of 5αR1 and 5αR2
Expression of 5αR1 mRNA, shown in Figure 5a, was greater in MCF-7, MDA-MB-231, and T-47D cells than in MCF-10A cells. Semi-quantitative RT-PCR analysis of 5αR1 (relative to 18S mRNA) gave values of 2.55 (± 0.10) for MCF-7 cells, 2.13 (± 0.17) for MDA-MB-231 cells, 2.76 (± 0.22) for T-47D cells and 1.09 (± 0.12) for MCF-10A cells (Fig. 5a, right panel). This represents significant (p < 0.001) 2.34-, 1.95-, and 2.53-fold higher expression, respectively, for MCF-7, MDA-MB-231 and T-47D cells than for MCF-10A cells. RT-PCR analysis for 5αR2 showed statistically insignificant differences between cell types (Fig. 5b). The mean (± SEM) of 5αR2/18S ratios (n = 5–6) were 0.74 (± 0.05), 0.59 (± 0.07), 0.81 (± 0.10), and 0.87 (± 0.11) for MCF-7, MDA-MB-231, T-47D and MCF-10A cells, respectively.
Figure 5.
Representative gels showing the relative expression of (a) 5αR1, (b) 5αR2, (c) 20α-HSO, (d) 3α-HSO type 2, and (e) 3α-HSO type 3 in MCF-7 (lane 2), MDA-MB-231 (lane 3), T-47D (lane 4), and MCF-10A (lane 5) cells. In each case aliquots of cDNA were amplified with gene-specific primers, run on 9% polyacrylamide gels and the bands were quantified as described under Methods. Each right panels shows computer-assisted quantification of data calculated as ratios against 18S rRNA (mean ± SEM) from 6 separate experiments. *** significantly different from MCF-7, MDA-MB-231, and T-47D cells at p < 0.001.
Expression of 20α-HSO, 3α-HSD2, 3α-HSD3 and 3β-HSD
A representative gel showing 20α-HSO (AKR1C1) expression in the four cell types is shown in Figure 5c. The ratio of 20α-HSO to 18S rRNA (n = 6) was 1.40 (± 0.10), 3.00 (± 0.24), 0.96 (± 0.10) and 8.59 (± 0.91) in the MCF-7, MDA-MB-231, T-47D and MCF-10A cells respectively. This represents a significant (p < 0.001) 6.1-, 2.9- and 8.9-fold greater expression of 20αHSO in MCF-10A cells than in MCF-7, MDA-MB-231, and T-47D cells, respectively.
We were unable to detect mRNA expression of 3α-HSO type 1 (AKR1C4) in any of the four cell types. A representative gel showing 3α-HSO2 (AKR1C3) expression in the four cell types is shown in Figure 5d. The ratio of 3α-HSO2 to 18s rRNA (n = 6) was 0.38 (± 0.30), 4.12 (± 0.26), 0.82 (± 0.09) and 6.87 (± 0.74) in the MCF-7, MDA-MB-231, T-47D and MCF-10A cells, respectively. This represents significant (p < 0.001) 18.1-, 1.6- and 8.4-fold greater expression of 3α-HSO2 in MCF-10A cells than in MCF-7, MDA-MB-231, and T-47D cells, respectively. The expression of 3α-HSO2 in MDA-MB-231 cells was also significantly (p < 0.001) higher than in MCF-7 and T-47D cells.
A representative gel showing 3α-HSO3 (AKR1C2) expression in the four cell types is shown in Figure 5e. The ratio of 3α-HSO3 was 1.59 (± 0.19), 0.26 (± 0.03), 1.54 (± 0.17) and 17.18 (0.95) in the MCF-7, MDA-MB-231, T-47D and MCF-10A cells, respectively. This represents 10.8-, >20- and 11.2-fold greater expression (p < 0.001) of 3α-HSO3 in MCF-10A cells than in MCF-7, MDA-MB-231 and T-47D cells, respectively.
3β-HSD Type 1 (HSD3B1) was expressed in all cells but the levels were inconsistent for reliable quantification (results not shown). 3β-HSO type 2 expression was not detected in any of the four cell types.
Discussion
Previous in vitro metabolism studies have shown that mammary tissue from mouse [18], rat [19-21] and human [1,22,23] can convert progesterone to metabolites whose formation would require the action of the enzymes, 5αR, 3α-HSO, 3β-HSO and 20α-HSO. The results of the current investigation show that MCF-7, MDA-MB-231, T-47D and MCF-10A human breast cell lines retain the activities of these progesterone metabolizing enzymes. An outline of the major metabolic pathways and the positions of the enzymes in the breast cell lines is presented in Figure 6. Of interest is that these pathways appear to be the same as those found in breast tissues [1]. Moreover, differences between tumorous and nontumorous breast tissue in terms of relative activities of enzymes such as 5αR and 20α-HSO [1,18,19,23] were observed between tumorigenic and nontumorigenic cell lines in the present study.
5α-Reductase
The enzyme responsible for the conversion of 4-ene steroids to 5α-reduced steroids is 4-ene-steroid 5α-reductase, known commonly as 5α-reductase (5αR; EC 1.3.99.5) [24]. There are two known isoforms of the human 5α-reductase, namely type 1 (5αR1) and type 2 (5αR2) [24-26]. 5αR1, which is encoded by the SRD5A1 gene and is composed of 259 amino acids, has an optimum pH of 6–9, whereas 5αR2, encoded by the SRD5A2 gene, and composed of 254 amino acids, has an optimum pH of 5.5 [27]. 5αR1 has been detected in various androgen-independent organs, such as the liver and brain [28]. 5αR2 has been found predominantly in androgen-dependent organs, such as epididymis and prostate [24,28] and its role in prostate cancer has been extensively studied. Recently 5αR1 and 5αR2 were located in human breast carcinoma and were studied in relation to 5α-reduction of testosterone [29]. Although several studies have recently examined SRD5A2 polymorphisms in association with breast cancer [30-32], to date no study has addressed the relative expression of 5αR1 and 5αR2 in tumorous and normal breast tissues or breast cell lines.
Tumorous mammary gland tissue has a greater ability to convert progesterone to 5α-reduced metabolites (5α-pregnanes) than nontumorous tissue [1,18,19,23]. The results of the present study provide the first demonstration that MCF-7, MDA-MB-231 and T-47D human breast cell lines have significantly greater ability to convert progesterone to 5α-pregnanes than the MCF-10A human breast cell line. Conversion of progesterone to 5α-pregnanes is the result of 5α-reductase activity. The quantitative differences in 5α-reductase activities between cell lines do not appear to be related to the presence or absence of ER or PR, since significantly higher (1.9–3.3-fold) 5α-reductase activity was evident in MCF-7 and T-47D (ER-positive and PR-positive) as well as MDA-MB-231 (ER-negative and PR-negative) cells than in the ER/PR-negative MCF-10A cells. The one consistent difference is that MCF-7, MDA-MB231 and T-47D cells will form tumors [5,6], whereas MCF-10A will not form tumors [4] in immunodeficient mice. These results from cell lines are consistent with results from matched breast tissues of patients in which tumorous tissue exhibited significantly higher progesterone 5α-reductase activity than nontumorous tissue, regardless of presence or absence of ER and/or PR [1]. The 5α-pregnanes:4-pregnenes ratio was about 8-fold higher in tumorous than in nontumorous breast tissue after an 8-hour incubation with [14C]progesterone [1]. Studies with breast cell lines, showing that 5α-pregnanes stimulate proliferation and decrease attachment of cells [1,2] prompted us to suggest that neoplasia in human breast may be promoted by increases in ratio of 5α-pregnanes:4-pregnenes. In the current studies, the ratio of 5α-pregnanes:4-pregnenes in the tumorigenic cells was 6.5-fold higher for MCF-7 cells, 9.6-fold higher for MDA-MB-231 cells, and 7.1-fold higher for T-47D cells than for the nontumorigenic MCF-10A cells. Therefore, both tissue and breast cell line studies suggest that an elevated level of progesterone 5α-reductase activity may be an indicator of breast tumorigenesis, regardless of presence or absence of ER and/or PR. However, it should be noted that only a single nontumorous cell line was examined and it will be necessary to study other "normal" breast cell lines before making generalizations regarding the relationship between changes in steroid enzyme activity and tumorigenicity in human breast cell lines.
Several factors can account for increases in 5α-reductase activity. In vivo, increases in enzyme activity can result from increased synthesis of enzyme due to increased expression of the mRNA coding for the enzyme, or from changes in the milieu in which the enzymes operate (such as temperature and pH, and concentrations of cofactors, substrates, products, competitors, ions, phospholipids and other molecules). In in vitro experiments, the milieu is carefully controlled to be constant for all the incubations and therefore observed differences can be more easily ascribed to differences in enzyme amounts resulting from increased expression. The results of the RT-PCR studies show that the expression of 5αR1 is significantly greater in MCF-7, MDA-MB-231 and T-47D cells than in MCF-10A cells. Although 5αR2 is expressed approximately equally in the four cell types, the abundance of 5αR1 mRNA transcripts greatly exceeds that of the 5αR2 transcripts. Using identical PCR conditions it required 8–10 more PCR cycles to amplify a 5αR2 band to the intensity of a 5αR1 band in each of the cell types. Since each cycle theoretically results in a doubling of PCR product, 5αR1 mRNA appears to be present at levels in the range of about 250–1000 fold higher than the 5αR2 mRNA. Although this can only be considered a qualitative observation, it appears reasonable to conclude that 5αR1 mRNA represents the predominant 5αR mRNA present in each of the four cell types tested here. Further, the differences in expression of 5αR1 (Fig. 5a) between cell types (high in MCF-7, MDA-MB-231 and T-47D cells and low in MCF-10A cells) in general parallel the differences in total 5α-reductase activities (Fig. 2). It was also recently demonstrated by immunohistochemistry and RT-PCR that 5αR1 is the main isoform expressed in human breast carcinomas [29] and that 5αR2 may not be associated with risk of breast cancer [30-32]. These observations provide strong evidence that 5αR1 is the primary 5α-reductase expressed in these cell lines and that the differences in 5α-pregnane production between the cells is due primarily to a difference in 5αR1 expression. This does not suggest, however, that 5αR2 may not play any role in progesterone metabolism in these cells under certain limiting conditions. Differences in biochemical [33] and pharmacological properties [34] and tissue pattern of expression [28,33], and affinities for substrates [33,35], have suggested distinct physiological functions for 5αR1 and 5αR2. As in the case of 5α-reductase activity, the presence or absence of ER and PR do not appear to be related to 5α-reductase expression.
The role of 5α-reduction of androgens in prostatic hyperplasia [36] and carcinoma [37] as it relates to 5α-dihydrotestosterone production [38] and 5α-reductase gene expression [39] are well recognized. The potential importance of 5α-reduction of progesterone in breast cancer has only recently received attention [1]. The studies presented here on breast cell lines further suggest a link between tumorigenicity and increased 5α-reductase activity. If the 5α-pregnanes resulting from progesterone 5α-reductase activity are involved in the risk and development of breast cancer and if local 5α-reduction of progesterone can influence or be influenced by the behavior of tumors, then changes in expression of 5α-reductase may have important implications for the prevention, therapy and biological understanding of breast cancer. Moreover, tumorigenic and nontumorigenic breast cell lines may provide a useful in vitro system for studying the control of 5α-reductase activity and expression in breast cancer.
The 20α – and 3α-(3β)-Hydroxysteroid Oxidoreductases
The formation of progesterone metabolites by breast tissue also involves the activities of 3α-HSO, 20α-HSO and 3β-HSO, as evidenced by the formation of the metabolites: 20α-hydoxy-4-pregnen-3-one (20αDHP), 20α-hydroxy-5α-pregnan-3-one, 3α-hydroxy-4-pregnen-20-one (3αHP), 3α-hydroxy-5α-pregnan-20-one, 3β-hydroxy-5α-pregnan-20-one, 4-pregnene-3α,20α-diol, and 5α-pregnane-3α (β),20α-diol.
20α-HSO is responsible for the reductive/oxidative interconversions of progesterone and 20αDHP, 3αHP and 4-pregnene-3α,20α-diol, 5α-pregnane-3,20-dione and 20α-hydroxy-5α-pregnan-3-one, and 5α-pregnan-3α (β)-ol-20-one and 5α-pregnane-3α (β),20α-diol. Our report shows that the 20α-HSO activity was significantly higher in MCF-10A cells than in the three other cell lines and that this activity resulted primarily in 4-pregnene-20α-hydroxy steroids (20αDHP and 4-pregnene-3α,20α-diol). Higher levels of 20α-HSO activity have been reported in normal human [1] and rat [19] mammary gland tissue than in respective tumorous tissues. Human 20α-HSO is mainly associated with microsomes [40]. Its characterization from human skin [41] showed that human 20α-HSO preferentially catalyzes the reduction of progesterone to 20αDHP with relatively low reverse (oxidative) activity.
Human 20α-HSO is a member of the aldo-keto reductase (AKR) gene superfamily [42,43]. Expression of 20α-HSO mRNA has been demonstrated in a number of tissues, including mammary gland [41]. AKRs are monomeric 37 kDa proteins and are NAD(P)(H)-dependent [44]. The isoform AKR1C1 is predominantly a 20α-HSO [42] although the isoforms AKR1C2-AKR1C4 are also able to reduce progesterone to 20αDHP and oxidize 20αDHP to progesterone [43]. The relative abundance of these isoforms in MCF-7, MDA-MB-231, T-47D and MCF-10A cells was investigated in our studies by RT-PCR and showed significantly higher expression of AKR1C1, AKR1C2 and AKR1C3 mRNA in MCF-10A than in the other cells. These higher levels of expression suggest that the higher 20α-HSO activity observed in the MCF-10A cell progesterone metabolism studies is due to higher levels of AKR1C1 (and perhaps the other AKR isozyme) mRNA(s).
Human 3α-HSO is responsible for the interconversions of 3-oxo and 3α-hydroxy steroids and in the case of progesterone metabolism, results in interconversions between progesterone and 3αHP, 20αDHP and 4-pregnene-3α,20α-diol, 5α-pregnane-3,20-dione and 3α-hydroxy-5α-pregnan-20-one, 20α-hydroxy-5α-pregnan-3-one and 5α-pregnan-3α,20α-diol. The 3α-HSO activities for MCF-7, MDA-MB-231 and T-47D cells (5–10 pmoles/106 cells/hour) were about 2.5–5.5-fold lower than the 3α-HSO activity of MCF-10A cells. Interestingly, in matched breast tissue samples [1] the 3α-reductive activity was 2.9- to 3.3-fold higher in normal (nontumorous) than in tumorous breast tissues [1]. Using RT-PCR we showed that the three isoforms AKR1C1–AKR1C3 are expressed in each of the four cell lines examined and that expression of each was highest in MCF-10A cells. In human, all three isoforms have been shown to be able to act as NAD(P)(H)-dependent 3α-oxidoreductases, with AKR1C2 and AKR1C3 predominating in 3α-HSO activity [43,45] in prostate and mammary glands [43]. A fourth isoform, AKR1C4 (3α-HSO type 1), was not expressed in any of the four cell lines, agreeing with previous findings using human breast tissue [42].
3β-HSO activities have been noted in human breast tissue [1] and in MCF-7 [46], T-47Dco [47] and ZR-75-1 [48] human breast cell lines. Our data also indicates 3β-HSO activity and mRNA expression of HSO3B1 (3β-HSO type 1) in MCF-7, MDA-MB-231, T-47D and MCF-10A breast cell lines but no significant differences between the cell types.
It has been suggested [49] that in hormone-related cancers (breast, prostate, endometrium, testis, ovary, thyroid and osteosarcoma) hormones, both endogenous and exogenous, provide the stimulus along the cancer progression pathway. Therefore anti-hormone therapies (such as tamoxifen) are effective in stopping the progression and thereby increase the time to recurrence and death. Unfortunately hormone-related cancers invariably become what has been called "hormone independent" and thereupon no longer respond to the anti-hormone therapies. Despite a large number of studies, "hormone independence" in cancer is poorly understood. Estradiol17β is considered the active hormone in most so-called hormone-related breast cancers, and "hormone independence" implies "estradiol independence". Our previous studies [1-3] and the current report on the differences in progesterone metabolism between tumorous and normal breast tissue, tumorigenic and nontumorigenic breast cell lines and significant actions of the progesterone metabolites on breast cell lines, regardless of ER and PR status, lead us to suggest that during the breast cancer progression pathway, change in progesterone metabolizing enzyme expression, and hence enzyme activity profile, occur in affected breast tissues. The resulting increases in tumor- and metastasis promoting – and concomitant decreases in inhibitory – progesterone metabolites may provide the stimulus for progression and malignancy of the tumor. Aspects of the hypothesis could be tested in vitro with breast cell lines and in vivo with immunodefient mice, using specific stimulatory and inhibitory agents for progesterone metabolizing enzyme activity and expression.
Conclusions
The findings provide the first evidence that the conversion of progesterone to the cancer promoting 5α-pregnanes is significantly higher in the human tumorigenic breast cell lines, MCF-7 (ER+/PR+), MDA-MB-231 (ER-/PR-) and T-47D (ER+/PR+), than in the nontumorigenic cell line, MCF-10A (ER-/PR-). Although both 5αR1 and 5αR2 are expressed by these cells, the elevated 5α-reductase activity appears to be the result of significantly greater expression of 5αR1. On the other hand, the activities of 3α-HSO and 20α-HSO, which result in direct conversion of progesterone to the cancer-inhibiting 3αHP and 20αDHP, are significantly higher in MCF-10A cells than in MCF-7, MDA-MB-231 and T-47D cells. The higher 3α-HSO and 20α-HSO activities correlate with significantly higher levels of 20α-HSO (AKR1C1), 3α-HSO2 (AKR1C3) and 3α-HSO3 (AKR1C2) mRNA expression in MCF-10A cells. Changes in progesterone metabolizing enzyme expression (resulting in enzyme activity changes) may be responsible for promoting breast cancer progression due to increased production of tumor-promoting 5α-pregnanes and decreased production of anti-cancer 20α – and 3α-4-pregnenes.
Authors' Contributions
Wiebe JP carried out and supervised the enzyme activity studies and drafted the manuscript. Lewis MJ carried out the expression studies and assisted in drafting the manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
This work was supported primarily by Grant No. BCTR 2000 805 from the Susan G. Komen Breast Cancer Foundation (U.S.A.) and some assistance from NSERC of Canada (Grant No. 6865). The technical assistance of Kelly Apostle, Guihua Zhang, Yousef P. Barbin and J. Glen MacDonald is gratefully acknowledged.
Contributor Information
John P Wiebe, Email: jwiebe@uwo.ca.
Michael J Lewis, Email: mjlewis@uwo.ca.
References
- Wiebe JP, Muzia D, Hu J, Szwajcer D, Hill SA, Seachrist JL. The 4-pregnene and 5α-pregnane progesterone metabolites formed in nontumorous and tumorous breast tissue have opposite effects on breast cell proliferation and adhesion. Cancer Res. 2000;60:936–943. [PubMed] [Google Scholar]
- Wiebe JP, Muzia D. The endogenous progesterone metabolite, 5α-pregnane-3,20-dione, decreases cell-substrate attachment, adhesion plaques, vinculin expression, and polymerized F-actin in MCF-7 breast cancer cells. Endocrine. 2001;16:7–14. doi: 10.1385/ENDO:16:1:07. [DOI] [PubMed] [Google Scholar]
- Weiler PJ, Wiebe JP. Plasma membrane receptors for the cancer-regulating progesterone metabolites, 5α-pregnane-3,20-dione and 3α-hydroxy-4-pregnen-20- one in MCF-7 breast cancer cells. Biochem Biophys Res Commun. 2000;272:731–737. doi: 10.1006/bbrc.2000.2847. [DOI] [PubMed] [Google Scholar]
- Soule HD, Maloney TM, Wolman SR, Peterson WD, Jr, Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF, Brooks SC. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990;50:6075–6086. [PubMed] [Google Scholar]
- Anderson WA, Perotti ME, McManaway M, Lindsey S, Eckberg WR. Similarities and differences in the ultrastructure of two hormone-dependent and one independent human breast carcinoma grown in athymic nude mice: comparison with the rat DMBA-induced tumor and normal secretory mammocytes. J Submicrosc Cytol. 1984;16:673–690. [PubMed] [Google Scholar]
- Soto AM, Murai JT, Siiteri PK, Sonnenschein C. Control of cell proliferation: evidence for negative control on estrogen-sensitive T47D human breast cancer cells. Cancer Res. 1986;46:2271–2275. [PubMed] [Google Scholar]
- Horwitz KB, Costlow ME, McGuire WL. MCF-7; a human breast cancer cell line with estrogen, androgen, progesterone, and glucocorticoid receptors. Steroids. 1975;26:785–795. doi: 10.1016/0039-128X(75)90110-5. [DOI] [PubMed] [Google Scholar]
- Read LD, Snider CE, Miller JS, Greene GL, Katzenellenbogen BS. Ligand-modulated regulation of progesterone receptor messenger ribonucleic acid and protein in human breast cancer cell lines. Mol Endocrinol. 1988;2:263–271. doi: 10.1210/mend-2-3-263. [DOI] [PubMed] [Google Scholar]
- Wiebe JP, Barr KJ, Buckingham KD. A radioimmunoassay for the regulatory allylic steroid, 3α-hydroxy-4-pregnen-20-one (3αHP) J Steroid Biochem Mol Biol. 1991;38:505–512. doi: 10.1016/0960-0760(91)90339-7. [DOI] [PubMed] [Google Scholar]
- Wiebe JP, Deline C, Buckingham KD, Dave V, Stothers JB. Synthesis of the allylic gonadal steroids, 3α-hydroxy-4-pregnen-20- one and 3α-hydroxy-4-androsten-17-one, and of 3α-hydroxy-5 alpha-pregnan-20-one. Steroids. 1985;45:39–51. doi: 10.1016/0039-128x(85)90064-9. [DOI] [PubMed] [Google Scholar]
- Wiebe JP, Dave V, Stothers JB. Synthesis and characteristics of allylic 4-pregnene-3,20-diols found in gonadal and breast tissues. Steroids. 1986;47:249–259. doi: 10.1016/0039-128x(86)90095-4. [DOI] [PubMed] [Google Scholar]
- Glasier MA, Wiebe JP, Hobkirk R. Progesterone metabolism by guinea pig intrauterine tissues. J Steroid Biochem Mol Biol. 1994;51:199–207. doi: 10.1016/0960-0760(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Wiebe JP, Boushy D, Wolfe M. Synthesis, metabolism and levels of the neuroactive steroid, 3α-hydroxy-4-pregnen-20-one (3αHP), in rat pituitaries. Brain Res. 1997;764:158–166. doi: 10.1016/S0006-8993(97)00452-6. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chen W, Zouboulis CC, Fritsch M, Blume-Peytavi U, Kodelja V, Goerdt S, Luu-The V, Orfanos CE. Evidence of heterogeneity and quantitative differences of the type 1 5α-reductase expression in cultured human skin cells – evidence of its presence in melanocytes. J Invest Dermatol. 1998;110:84–89. doi: 10.1046/j.1523-1747.1998.00080.x. [DOI] [PubMed] [Google Scholar]
- Boehmer AL, Brinkmann AO, Nijman RM, Verleun-Mooijman MC, de Ruiter P, Niermeijer MF, Drop SL. Phenotypic variation in a family with partial androgen insensitivity syndrome explained by differences in 5α-dihydrotestosterone availability. J Clin Endocrinol Metab. 2001;86:1240–1246. doi: 10.1210/jcem.86.3.7333. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Zakrajsek BA. Effect of experimental treatment on housekeeping gene expression: validation by real-time, quantitative RT-PCR. J Biochem Biophys Methods. 2000;46:69–81. doi: 10.1016/S0165-022X(00)00129-9. [DOI] [PubMed] [Google Scholar]
- Mori M, Tamaoki BI. In vitro metabolism of progesterone in the mammary tumor and the normal mammary gland of GRS/A strain of mice and dependency of some steroid-metabolizing enzyme activities upon ovarian function. Eur J Cancer. 1980;16:185–193. doi: 10.1016/0014-2964(80)90150-4. [DOI] [PubMed] [Google Scholar]
- Mori M, Tominaga T, Tamaoki BI. Steroid metabolism in normal mammary gland and in the dimethylbenzanthracene-induced mammary tumor of rats. Endocrinology. 1978;102:1387–1397. doi: 10.1210/endo-102-5-1387. [DOI] [PubMed] [Google Scholar]
- Mori M, Tominaga T, Kitamura M, Saito T, Tamaoki B. Effect of oophorectomy on progesterone metabolism in DMBA-induced mammary tumours of the rat. Eur J Cancer. 1980;16:1373–1375. doi: 10.1016/0014-2964(80)90297-2. [DOI] [PubMed] [Google Scholar]
- Eechaute W, de Thibault de Boesinghe L, Lacroix E. Steroid metabolism and steroid receptors in dimethylbenz(a)anthracene-induced rat mammary tumors. Cancer Res. 1983;43:4260–4265. [PubMed] [Google Scholar]
- Lloyd RV. Studies on the progesterone receptor content and steroid metabolism in normal and pathological human breast tissues. J Clin Endocrinol Metab. 1979;48:585–593. doi: 10.1210/jcem-48-4-585. [DOI] [PubMed] [Google Scholar]
- Verma U, Kapur MM, Laumas KR. Characterization of progesterone receptors and metabolism of progesterone in the normal and cancerous human mammary gland. J Steroid Biochem. 1978;9:569–577. doi: 10.1016/0022-4731(78)90124-3. [DOI] [PubMed] [Google Scholar]
- Russell DW, Wilson JD. Steroid 5α-reductase: two genes/two enzymes. Annu Rev Biochem. 1994;63:25–61. doi: 10.1146/annurev.biochem.63.1.25. [DOI] [PubMed] [Google Scholar]
- Andersson S, Russell DW. Structural and biochemical properties of cloned and expressed human and rat steroid 5α-reductases. Proc Natl Acad Sci U S A. 1990;87:3640–3644. doi: 10.1073/pnas.87.10.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson S, Berman DM, Jenkins EP, Russell DW. Deletion of steroid 5α-reductase 2 gene in male pseudohermaphroditism. Nature. 1991;354:159–161. doi: 10.1038/354159a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DW, Berman DM, Bryant JT, Cala KM, Davis DL, Landrum CP, Prihoda JS, Silver RI, Thigpen AE, Wigley WC. The molecular genetics of steroid 5α-reductases. Recent Prog Horm Res. 1994;49:275–284. doi: 10.1016/b978-0-12-571149-4.50018-0. [DOI] [PubMed] [Google Scholar]
- Thigpen AE, Silver RI, Guileyardo JM, Casey ML, McConnell JD, Russell DW. Tissue distribution and ontogeny of steroid 5α-reductase isozyme expression. J Clin Invest. 1993;92:903–910. doi: 10.1172/JCI116665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Darnel AD, Akahira JI, Ariga N, Ogawa S, Kaneko C, Takeyama J, Moriya T, Sasano H. 5α-reductases in human breast carcinoma: possible modulator of in situ androgenic actions. J Clin Endocrinol Metab. 2001;86:2250–2257. doi: 10.1210/jcem.86.5.7444. [DOI] [PubMed] [Google Scholar]
- Spurdle AB, Hopper JL, Chen X, Dite GS, McCredie MR, Giles GG, Venter DJ, Southey MC, Purdie DM, Chenevix-Trench G. The steroid 5α-reductase type II TA repeat polymorphism is not associated with risk of breast or ovarian cancer in Australian women. Cancer Epidemiol Biomarkers Prev. 2001;10:1287–1293. [PubMed] [Google Scholar]
- Scorilas A, Bharaj B, Giai M, Diamandis EP. Codon 89 polymorphism in the human 5α-reductase gene in primary breast cancer. Br J Cancer. 2001;84:760–767. doi: 10.1054/bjoc.2000.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharaj B, Scorilas A, Giai M, Diamandis EP. TA repeat polymorphism of the 5α-reductase gene and breast cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:387–393. [PubMed] [Google Scholar]
- Normington K, Russell DW. Tissue distribution and kinetic characteristics of rat steroid 5α-reductase isozymes. Evidence for distinct physiological functions. J Biol Chem. 1992;267:19548–19554. [PubMed] [Google Scholar]
- Poletti A, Rabuffetti M, Martini L. Effect of suramin on the biological activity of the two isoforms of the rat 5α-reductase. Steroids. 1996;61:504–505. doi: 10.1016/0039-128X(96)81082-8. [DOI] [PubMed] [Google Scholar]
- Thigpen AE, Cala KM, Russell DW. Characterization of Chinese hamster ovary cell lines expressing human steroid 5α-reductase isozymes. J Biol Chem. 1993;268:17404–17412. [PubMed] [Google Scholar]
- Wilson JD. The pathogenesis of benign prostatic hyperplasia. Am J Med. 1980;68:745–756. doi: 10.1016/0002-9343(80)90267-3. [DOI] [PubMed] [Google Scholar]
- Bruchovsky N, Rennie PS, Batzold FH, Goldenberg SL, Fletcher T, McLoughlin MG. Kinetic parameters of 5α-reductase activity in stroma and epithelium of normal, hyperplastic, and carcinomatous human prostates. J Clin Endocrinol Metab. 1988;67:806–816. doi: 10.1210/jcem-67-4-806. [DOI] [PubMed] [Google Scholar]
- Iehle C, Delos S, Guirou O, Tate R, Raynaud JP, Martin PM. Human prostatic steroid 5α-reductase isoforms – a comparative study of selective inhibitors. J Steroid Biochem Mol Biol. 1995;54:273–279. doi: 10.1016/0960-0760(95)00134-L. [DOI] [PubMed] [Google Scholar]
- Bruchovsky N, Sadar MD, Akakura K, Goldenberg SL, Matsuoka K, Rennie PS. Characterization of 5α-reductase gene expression in stroma and epithelium of human prostate. J Steroid Biochem Mol Biol. 1996;59:397–404. doi: 10.1016/S0960-0760(96)00125-2. [DOI] [PubMed] [Google Scholar]
- Pollow K, Lubbert H, Boquoi E, Pollow B. Progesterone metabolism in normal human endometrium during the menstrual cycle and in endometrial carcinoma. J Clin Endocrinol Metab. 1975;41:729–737. doi: 10.1210/jcem-41-4-729. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Dufort I, Rheault P, Luu-The V. Characterization of a human 20α-hydroxysteroid dehydrogenase. J Mol Endocrinol. 2000;25:221–228. doi: 10.1016/S0303-7207(99)00208-7. [DOI] [PubMed] [Google Scholar]
- Dufort I, Soucy P, Labrie F, Luu-The V. Molecular cloning of human type 3 3α-hydroxysteroid dehydrogenase that differs from 20α-hydroxysteroid dehydrogenase by seven amino acids. Biochem Biophys Res Commun. 1996;228:474–479. doi: 10.1006/bbrc.1996.1684. [DOI] [PubMed] [Google Scholar]
- Penning TM, Burczynski ME, Jez JM, Hung CF, Lin HK, Ma H, Moore M, Palackal N, Ratnam K. Human 3α-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem J. 2000;351:67–77. doi: 10.1042/0264-6021:3510067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jez JM, Bennett MJ, Schlegel BP, Lewis M, Penning TM. Comparative anatomy of the aldo-keto reductase superfamily. Biochem J. 1997;326:625–636. doi: 10.1042/bj3260625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufort I, Labrie F, Luu-The V. Human types 1 and 3 3α-hydroxysteroid dehydrogenases: differential lability and tissue distribution. J Clin Endocrinol Metab. 2001;86:841–846. doi: 10.1210/jcem.86.2.7216. [DOI] [PubMed] [Google Scholar]
- Raju U, Sklarew RJ, Post J, Levitz M. Steroid metabolism in human breast cancer cell lines. Steroids. 1978;32:669–680. doi: 10.1016/0039-128X(78)90077-6. [DOI] [PubMed] [Google Scholar]
- Fennessey PV, Pike AW, Gonzalez-Aller C, Horwitz KB. Progesterone metabolism in T47Dco human breast cancer cells – I. 5α-pregnan-3β,6α-diol-20-one is the secreted product. J Steroid Biochem. 1986;25:641–648. doi: 10.1016/0022-4731(86)90006-3. [DOI] [PubMed] [Google Scholar]
- Theriault C, Labrie F. Multiple steroid metabolic pathways in ZR-75-1 human breast cancer cells. J Steroid Biochem Mol Biol. 1991;38:155–164. doi: 10.1016/0960-0760(91)90121-K. [DOI] [PubMed] [Google Scholar]
- Henderson BE, Feigelson HS. Hormonal carcinogenesis. Carcinogenesis. 2000;21:427–433. doi: 10.1093/carcin/21.3.427. [DOI] [PubMed] [Google Scholar]