Abstract
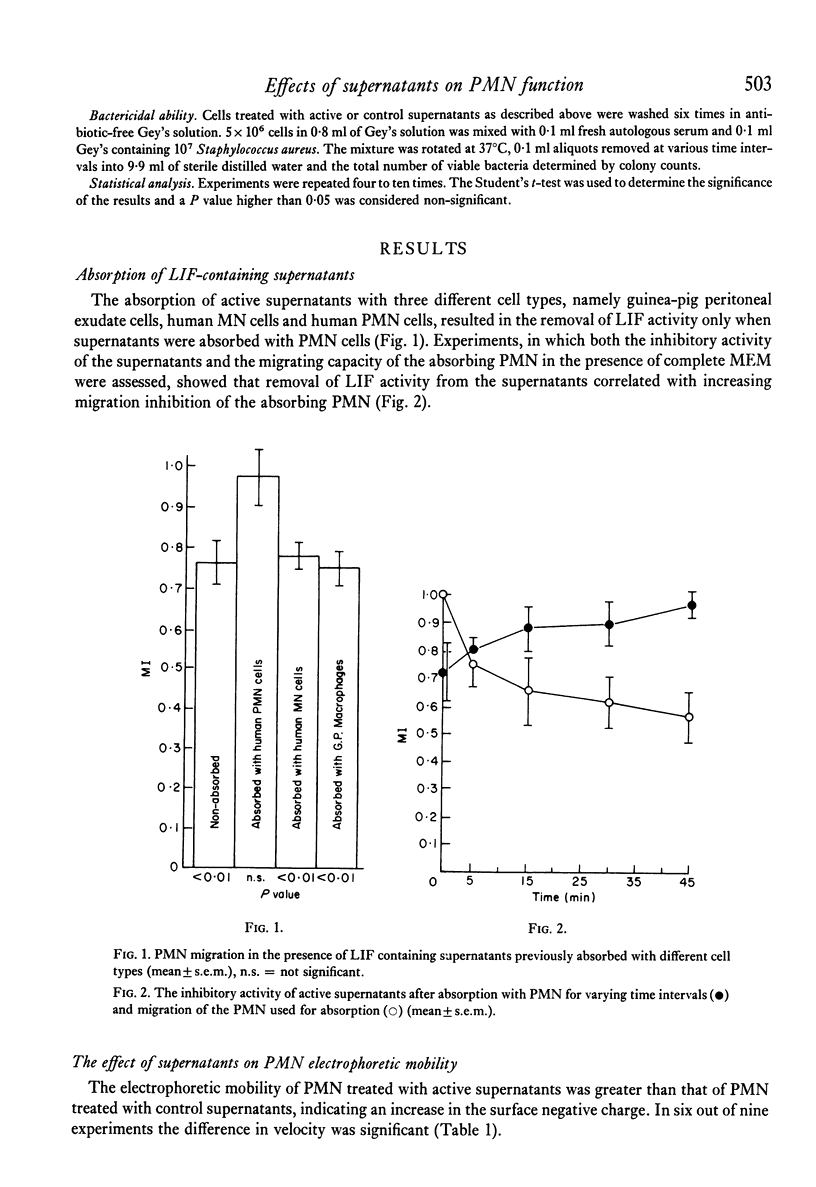
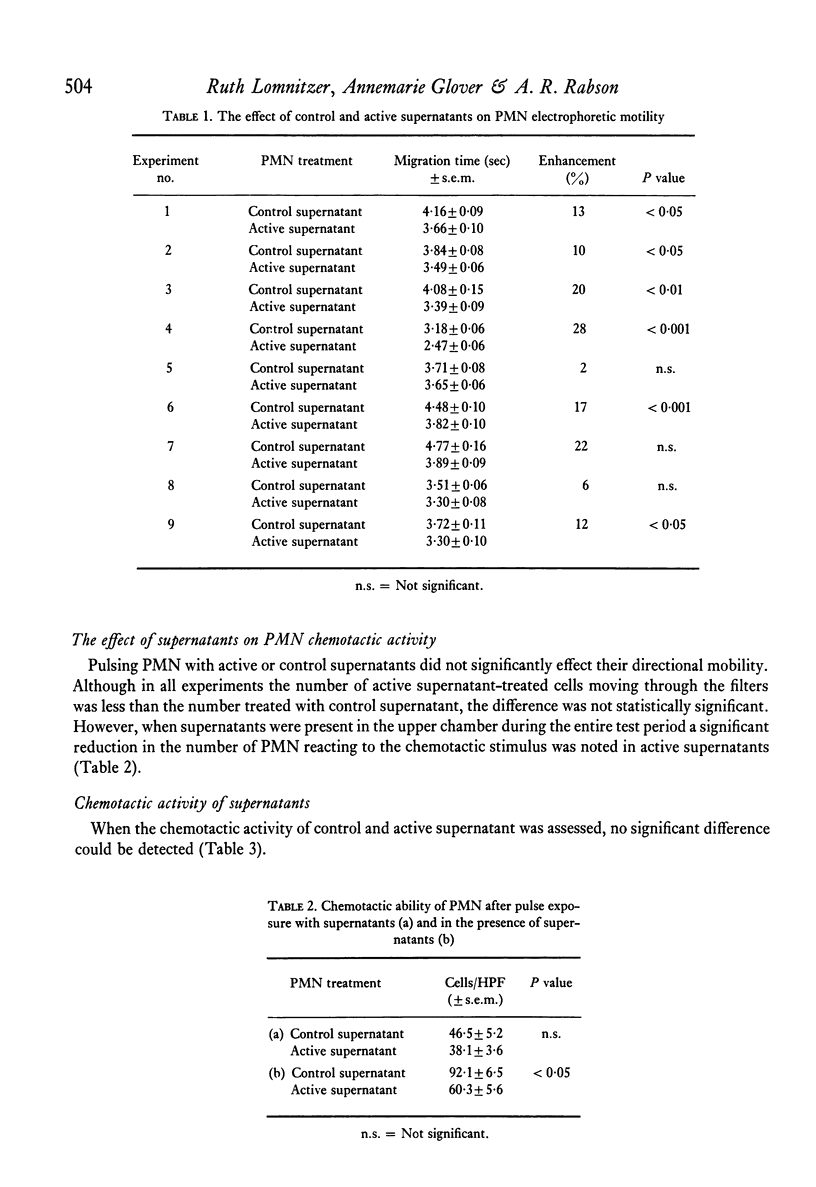
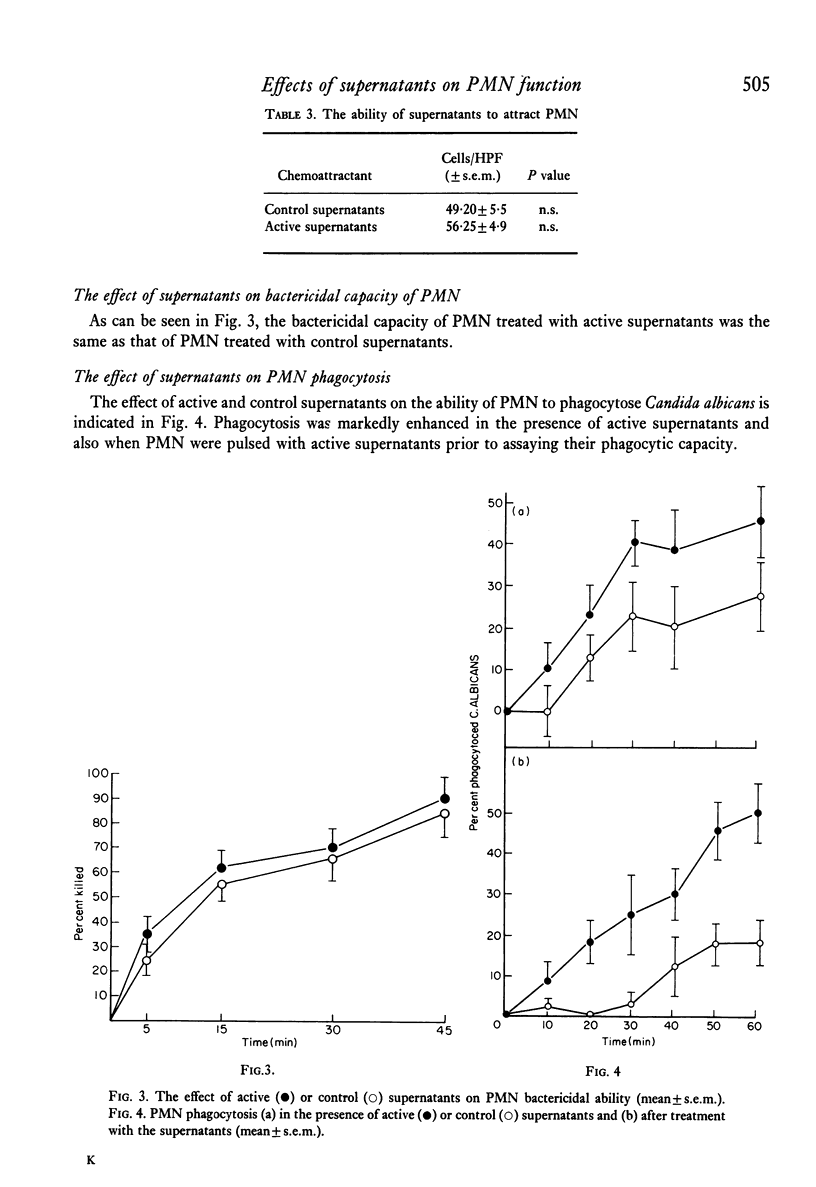
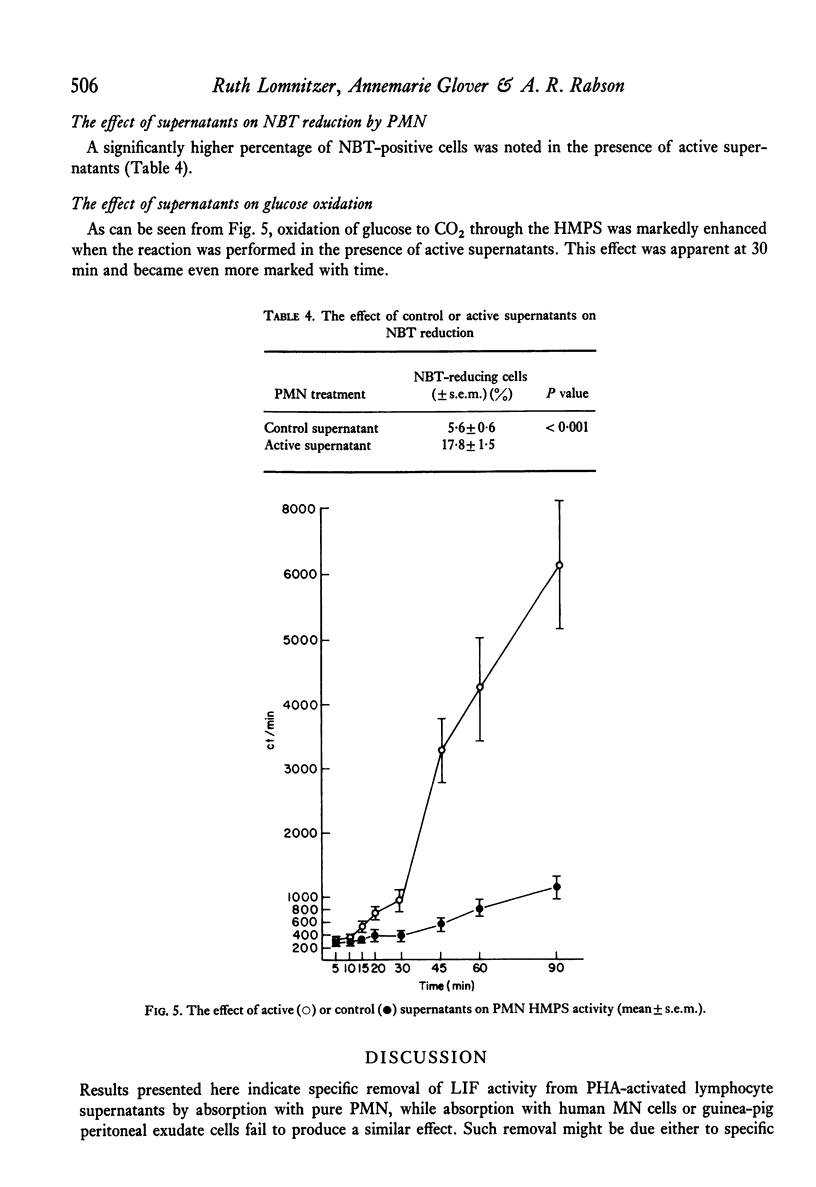
The effect of PHA-activated mononuclear-cell (MN) supernatants on various polymorphonuclear-leucocyte (PMN) functions were assessed. Treatment of PMN with PHA-activated MN-cell supernatants resulted in greater electrophoretic mobility, indicating an increase in the negative surface charge. PMN directional motility was inhibited in the presence of active supernatants but was not affected by a pulse exposure of the PMN to these supernatants. Neither control nor active supernatants were chemotactic for PMN, but treatment of these cells with active supernatants produced an increase in their phagocytic activity, their ability to reduce NBT and in their glucose oxidation through the hexosemonophosphate shunt. Bactericidal capacity of these PMN was unaltered. Specific loss of leucocyte inhibitory factor (LIF) activity from supernatants of PHA-activated MN cells followed their absorption with PMN cells but not with human MN cells or guinea-pig peritoneal exudate cells. Furthermore, acquired inhibition of migration of the absorbing PMN was observed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendtzen K. Inhibition of human leukocyte migration inhibitory factor (LIF) by alpha-L-fucose. Acta Allergol. 1975 Dec;30(6):327–338. doi: 10.1111/j.1398-9995.1975.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Bendtzen K. Some physicochemical properties of human leucocyte migration inhibitory factor (LIF). Acta Pathol Microbiol Scand C. 1976 Dec;84C(6):471–476. doi: 10.1111/j.1699-0463.1976.tb00057.x. [DOI] [PubMed] [Google Scholar]
- Cohen S. Cell mediated immunity and the inflammatory system. Hum Pathol. 1976 May;7(3):249–264. doi: 10.1016/s0046-8177(76)80036-6. [DOI] [PubMed] [Google Scholar]
- David J. R. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci U S A. 1966 Jul;56(1):72–77. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger G. G., Ogden B. E., Weston W. L. In vitro quantitation of cell-mediated immunity in guinea-pigs by macrophage reduction of nitro-blue tetrazolium. Clin Exp Immunol. 1976 Mar;23(3):517–524. [PMC free article] [PubMed] [Google Scholar]
- Leu R. W., Eddleston A. L., Hadden J. W., Good R. A. Mechanism of action of migration inhibitory factor (MIF). I. Evidence for a receptor for MIF present on the peritoneal macrophage but not on the alveolar macrophage. J Exp Med. 1972 Sep 1;136(3):589–603. doi: 10.1084/jem.136.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomnitzer R., Rabson A. R., Koornhof H. J. Leucocyte capillary migration: an adherence dependent phenomenon. Clin Exp Immunol. 1976 Aug;25(2):303–310. [PMC free article] [PubMed] [Google Scholar]
- Lomnitzer R., Rabson A. R., Koornhof H. J. The effects of cyclic AMP on leucocyte inhibitory factor (LIF) production and on the inhibition of leucocyte migration. Clin Exp Immunol. 1976 Apr;24(1):42–48. [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Karnovsky M. L., David J. R. Alterations of macrophage functions by mediators from lymphocytes. J Exp Med. 1971 Jun 1;133(6):1356–1376. doi: 10.1084/jem.133.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Remold H. G., David J. R. Characterization of a lymphocyte factor which alters macrophage functions. J Exp Med. 1973 Feb 1;137(2):275–290. doi: 10.1084/jem.137.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OREN R., FARNHAM A. E., SAITO K., MILOFSKY E., KARNOVSKY M. L. Metabolic patterns in three types of phagocytizing cells. J Cell Biol. 1963 Jun;17:487–501. doi: 10.1083/jcb.17.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven E. P., Axline S. G. Subplasmalemmal microfilaments and microtubules in resting and phagocytizing cultivated macrophages. J Cell Biol. 1973 Oct;59(1):12–27. doi: 10.1083/jcb.59.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocklin R. E. Partial characterization of leukocyte inhibitory factor by concanavalin A-stimulated human lymphocytes (LIF Con A). J Immunol. 1975 Apr;114(4):1161–1165. [PubMed] [Google Scholar]
- Rocklin R. E. Products of activated lymphocytes: leukocyte inhibitory factor (LIF) distinct from migration inhibitory factor (MIF). J Immunol. 1974 Apr;112(4):1461–1466. [PubMed] [Google Scholar]
- Rocklin R. E. Role of monosaccharides in the interaction of two lymphocyte mediators with their target cells. J Immunol. 1976 Mar;116(3):816–820. [PubMed] [Google Scholar]
- Ruthlomnitzer, Rabson A. R., Koornhof H. J. Production of leucocyte inhibitory factor (LIF) and macrophage inhibitory factor (MIF) by PHA-stimulated lymphocytes. Clin Exp Immunol. 1975 Dec;22(3):522–527. [PMC free article] [PubMed] [Google Scholar]
- Sher R., Anderson R., Rabson A. R., Koornhof H. J. Standardisation of the nitro-blue tetrazolium test and factors affecting its clinical application. S Afr Med J. 1974 Feb 9;48(6):209–212. [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Migration inhibitory factor and macrophage bactericidal function. Infect Immun. 1972 Aug;6(2):101–103. doi: 10.1128/iai.6.2.101-103.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrasher S. G., Yoshida T., Van Oss C. J., Cohen S., Rose N. R. Alteration of macrophage interfacial tension by supernatants of antigen-activated lymphocyte cultures. J Immunol. 1973 Feb;110(2):321–326. [PubMed] [Google Scholar]
- Tizard I. R., Holmes W. L. Phagocytosis of sheep erythrocytes by macrophages. Some observations under the scanning electron microscope. J Reticuloendothel Soc. 1974 Feb;15(2):132–138. [PubMed] [Google Scholar]
- Ward P. A., Remold H. G., David J. R. The production by antigen-stimulated lymphocytes of a leukotactic factor distinct from migration inhibitory factor. Cell Immunol. 1970 Jul;1(2):162–174. doi: 10.1016/0008-8749(70)90003-1. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Kuratsuji T., Takada A., Takada Y., Minowada J., Cohen S. Lymphokine-like factors produced by human lymphoid cell lines with B or T cell surface markers. J Immunol. 1976 Aug;117(2):548–554. [PubMed] [Google Scholar]


