Abstract
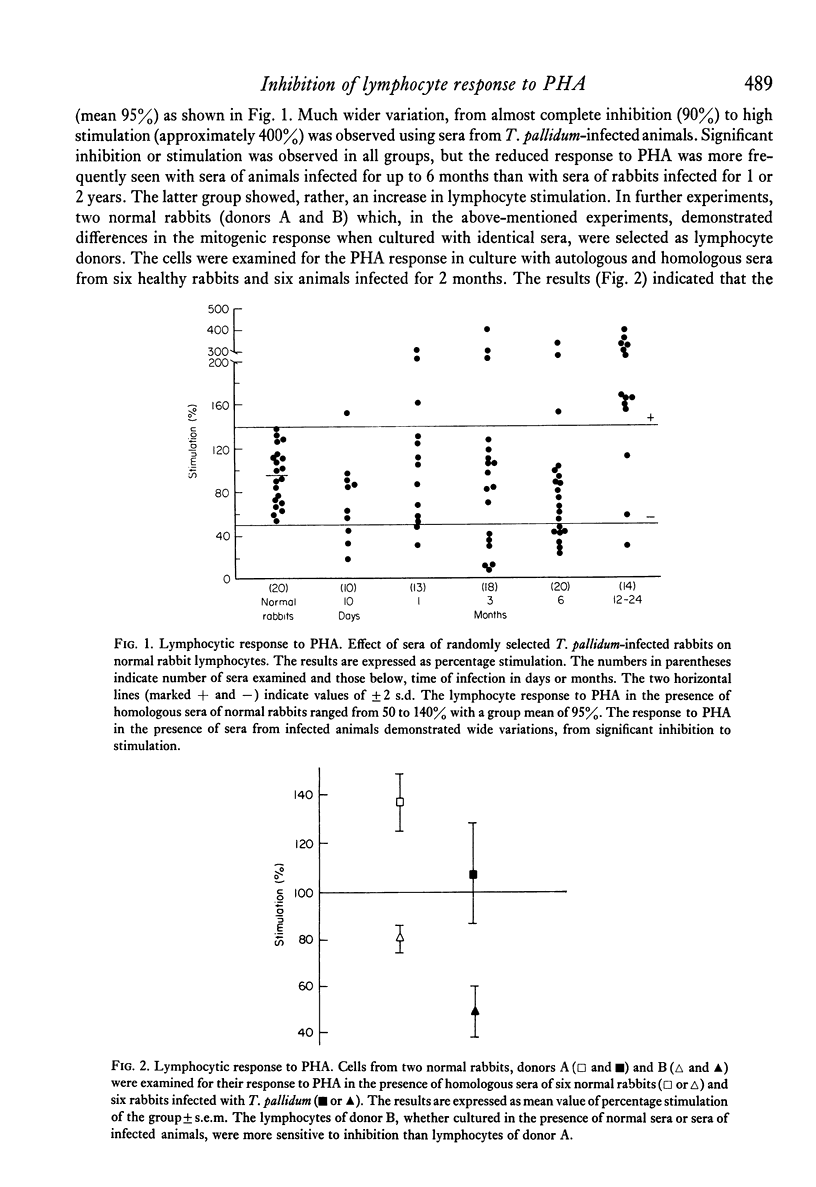
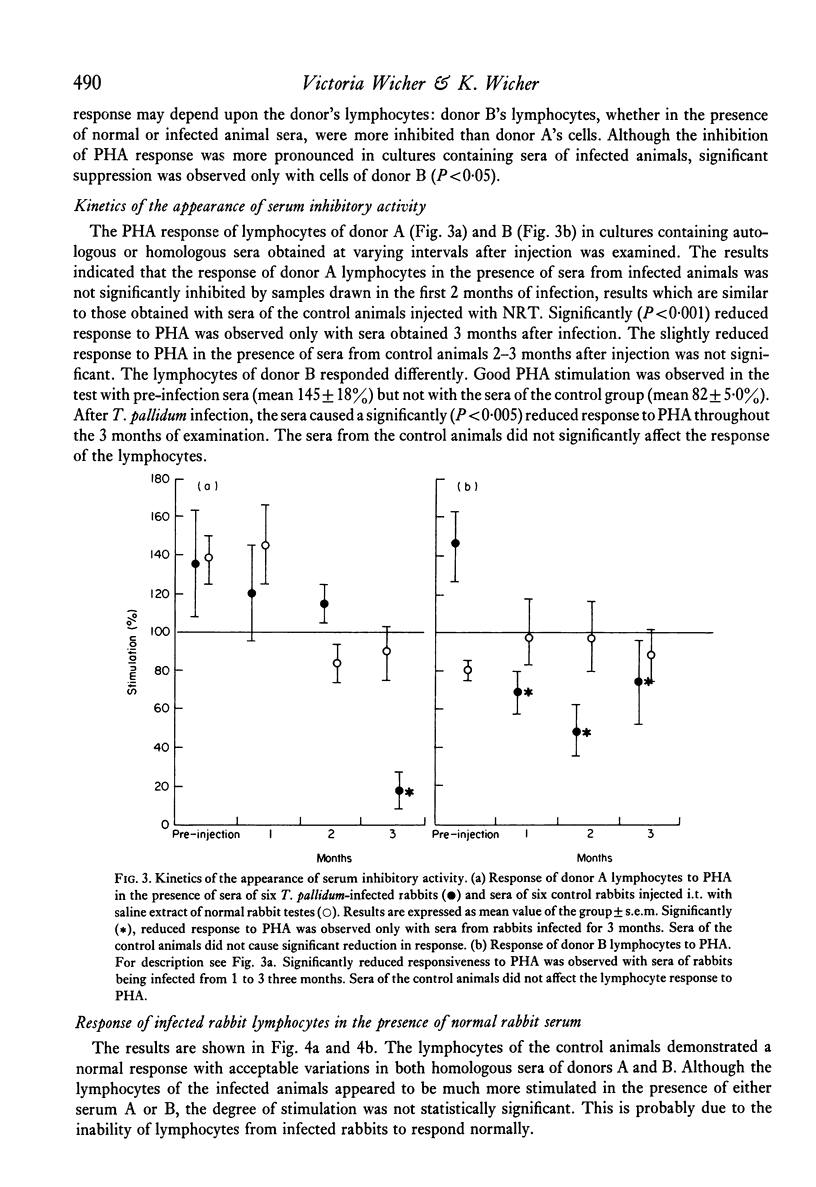
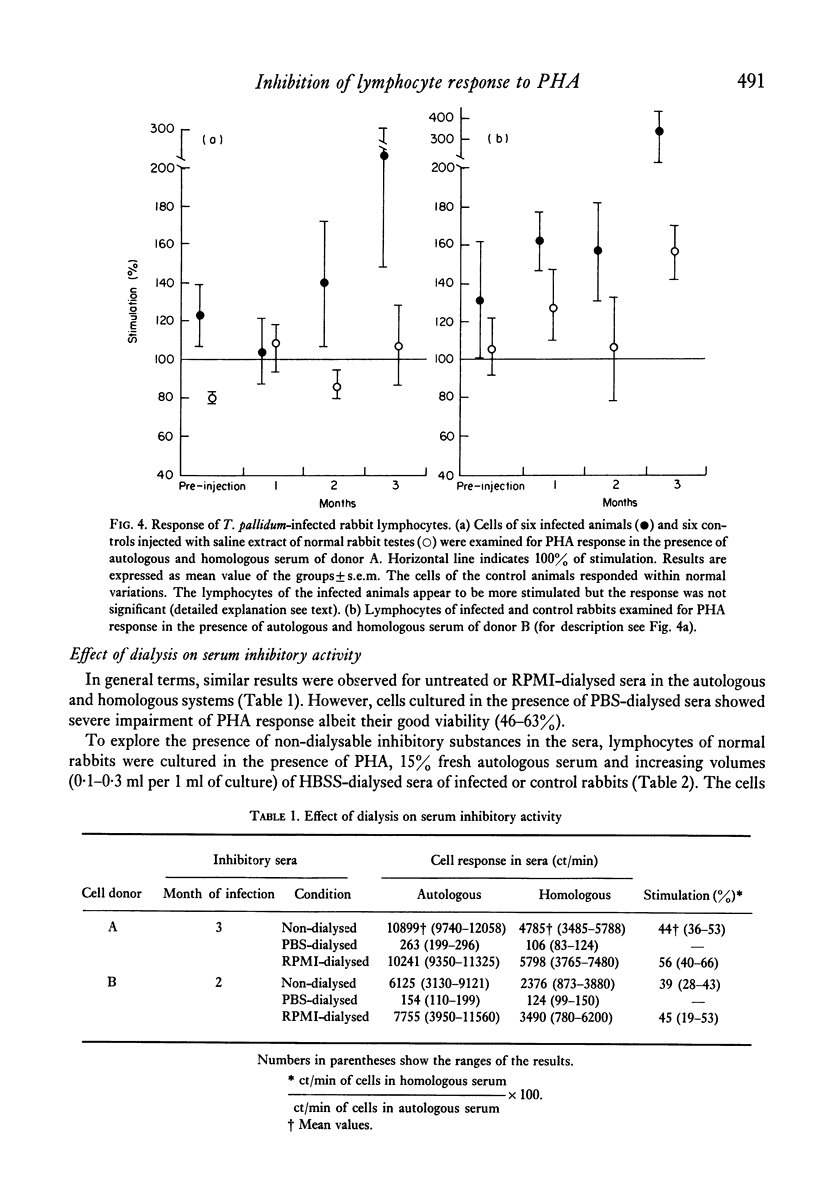
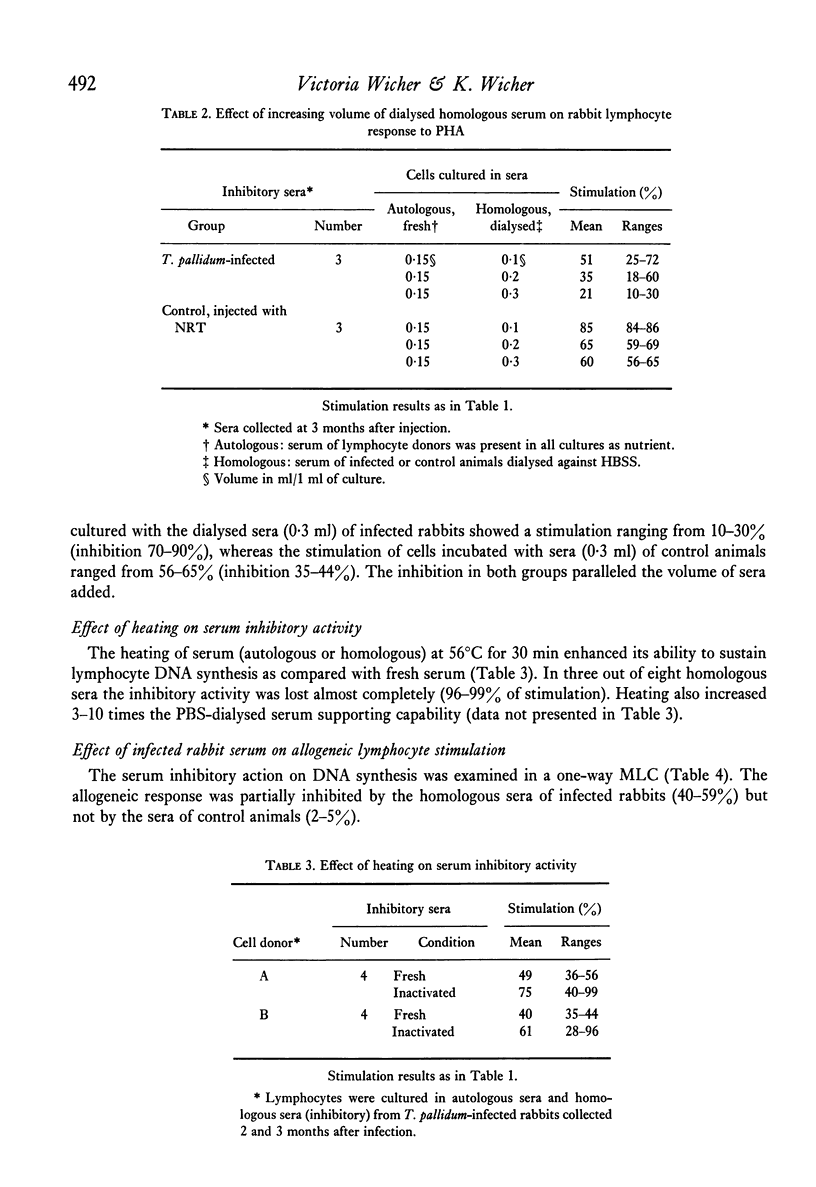
Serum inhibitors of lymphocyte response to PHA were found in T. pallidum-infected rabbits. The humoral inhibitors could be detected as early as 10 days after infection and persisted for at least 6 months. The factors also suppressed the allogeneic lymphocyte response. Control, or normal, rabbit sera likewise contain serum inhibitors, but in much lower concentration. The detection of the humoral inhibitors depended on the susceptibility of the indicator lymphocytes. Cells of some rabbits were more sensitive to the inhibitors than others. In addition to serum inhibitors, lymphocytes of T. pallidum-infected animals seem to be impaired and responded to PHA less vigorously than cells of normal rabbits. The inhibitory activity is most likely the result of a complex group of substances with different physicochemical characteristics; some pre-exist and others are newly formed after infection. Problems associated with the detection of such inhibitors are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alford R. H. Metal cation requirements for phytohemagglutinin-induced transformation of human peripheral blood lymphocytes. J Immunol. 1970 Mar;104(3):698–703. [PubMed] [Google Scholar]
- Bredt A. B., Mardiney M. R., Jr Reactivity of human lymphocytes to autologous and homologous serum. J Immunol. 1969 Jun;102(6):1526–1529. [PubMed] [Google Scholar]
- Bullock W. E., Jr, Fasal P. Studies of immune mechanisms in leprosy. 3. The role of cellular and humoral factors in impairment of the in vitro immune response. J Immunol. 1971 Apr;106(4):888–899. [PubMed] [Google Scholar]
- Field E. J., Shenton B. K. Inhibitory effect of unsaturated fatty acids on lymphocyte-antigen interaction with special reference to multiple sclerosis. Acta Neurol Scand. 1975 Aug;52(2):121–136. doi: 10.1111/j.1600-0404.1975.tb05766.x. [DOI] [PubMed] [Google Scholar]
- Ford W. H., Caspary E. A., Shenton B. Purification and properties of a lymphocyte inhibition factor from human serum. Clin Exp Immunol. 1973 Oct;15(2):169–179. [PMC free article] [PubMed] [Google Scholar]
- Forsdyke D. R. Serum factors affecting the incorporation of (3H)thymidine by lymphocytes stimulated by antigen. 3. Evidence for a role of complement from studies with specific complement inhibitors. Immunology. 1973 Oct;25(4):613–619. [PMC free article] [PubMed] [Google Scholar]
- Knowles M., Hughes D., Caspary E. A., Field E. J. Lymphocyte transformation in multiple sclerosis. Inhibition of unstimulated thymidine uptake by a serum factor. Lancet. 1968 Dec 7;2(7580):1207–1209. doi: 10.1016/s0140-6736(68)91691-7. [DOI] [PubMed] [Google Scholar]
- Levene G. M., Turk J. L., Wright D. J., Grimble A. G. Reduced lymphocyte transformation due to a plasma factor in patients with active syphilis. Lancet. 1969 Aug 2;2(7614):246–247. doi: 10.1016/s0140-6736(69)90010-5. [DOI] [PubMed] [Google Scholar]
- Mangi R. J., Dwyer J. M., Kantor F. S. The effect of plasma upon lymphocyte response in vitro. Demonstration of a humoral inhibitor in patients with sarcoidosis. Clin Exp Immunol. 1974 Dec;18(4):519–528. [PMC free article] [PubMed] [Google Scholar]
- Morse J. H. Immunological studies of phytohaemagglutinin. I. Reaction between phytohaemagglutinin and normal sera. Immunology. 1968 May;14(5):713–724. [PMC free article] [PubMed] [Google Scholar]
- Musher D. M., Schell R. F., Knox J. M. In vitro lymphocyte response to Treponema refringens im human syphilis. Infect Immun. 1974 Apr;9(4):654–657. doi: 10.1128/iai.9.4.654-657.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S., Folds J. D., Baseman J. B. Depression of lymphocyte response to concanavalin A in rabbits infected with Treponema pallidum (Nichols strain). Infect Immun. 1976 Jul;14(1):320–322. doi: 10.1128/iai.14.1.320-322.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki P. I., Mottironi V. D., Barnett E. V. Cytotoxins in disease. Autocytotoxins in lupus. N Engl J Med. 1970 Oct 1;283(14):724–728. doi: 10.1056/NEJM197010012831403. [DOI] [PubMed] [Google Scholar]
- Waksman B. H., Namba Y. On soluble mediators of immunologic regulation. Cell Immunol. 1976 Jan;21(1):161–176. doi: 10.1016/0008-8749(76)90337-3. [DOI] [PubMed] [Google Scholar]
- Wicher V., Wicher K. In vitro cell response of Treponema pallidum-infected rabbits. I. Lymphocyte transformation. Clin Exp Immunol. 1977 Sep;29(3):480–486. [PMC free article] [PubMed] [Google Scholar]
- Yachnin S., Raymond J. An absolute requirement for serum macromolecules in phytohaemagglutinin-induced human lymphocyte DNA synthesis. Clin Exp Immunol. 1975 Oct;22(1):153–166. [PMC free article] [PubMed] [Google Scholar]


