Abstract
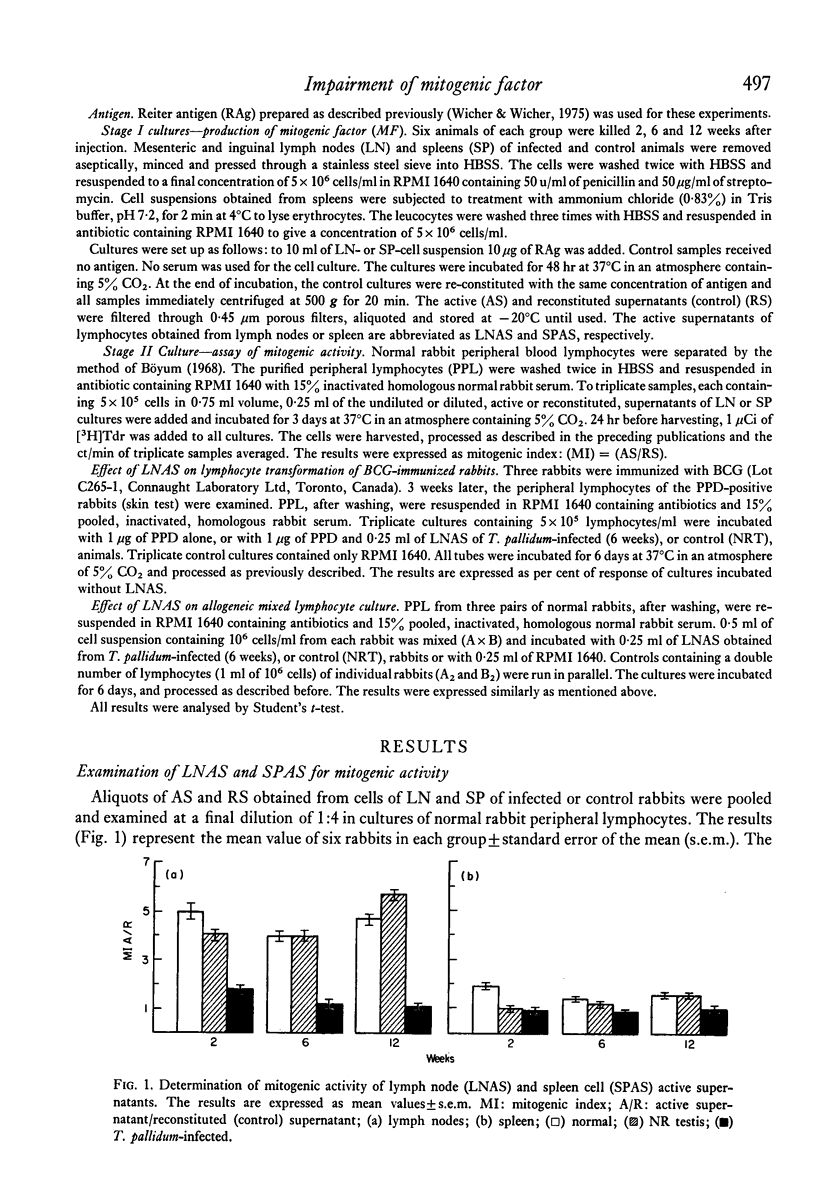
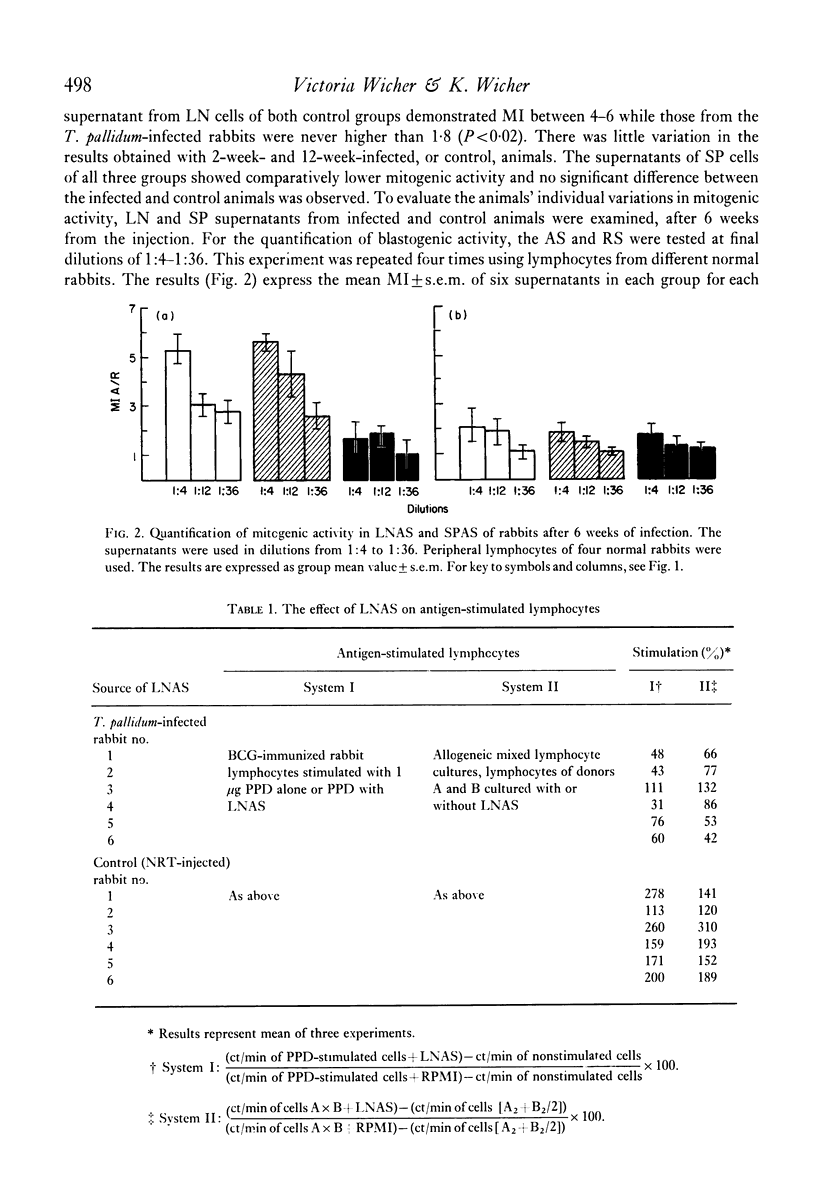
Production of mitogenic factor was examined in rabbits infected intratesticularly with T. pallidum and in control animals injected with saline or saline extract of normal rabbits' testes. Lymph nodes and spleen from animals killed 2, 6 and 12 weeks after injection were used as the source of lymphocytes, cultured in serum-free medium in the presence of Reiter antigen. The active supernatants of lymph node cells (LNAS) and spleen cells (SPAS) were examined for the presence of mitogenic factor using normal rabbit peripheral lymphocytes. The LNAS of control animals showed a mitogenic index (MI) between 4 and 6 and the infected animals less than 2. The SPAS of infected and control rabbits showed an MI of less than 2. The lower mitogenicity in LNAS of infected and that of SPAS of infected and control animals seems to be due to the presence of inhibitors of DNA synthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fulford K. W., Brostoff J. Leucocyte migration and cell-mediated immunity in syphilis. Br J Vener Dis. 1972 Dec;48(6):483–488. doi: 10.1136/sti.48.6.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha R. S., Merler E. Human lymphocyte mitogenic factor: synthesis by sensitized thymus-derived lymphocytes, dependence of expression on the presence of antigen. Cell Immunol. 1974 Jan;10(1):86–104. doi: 10.1016/0008-8749(74)90154-3. [DOI] [PubMed] [Google Scholar]
- Gershon R. K. A disquisition on suppressor T cells. Transplant Rev. 1975;26:170–185. doi: 10.1111/j.1600-065x.1975.tb00179.x. [DOI] [PubMed] [Google Scholar]
- Kasakura S. Heterogeneity of blastogenic factors produced in vitro by antigenically stimulated and unstimulated leukocytes. J Immunol. 1970 Nov;105(5):1162–1167. [PubMed] [Google Scholar]
- Musher D. M., Schell R. F., Knox J. M. In vitro lymphocyte response to Treponema refringens im human syphilis. Infect Immun. 1974 Apr;9(4):654–657. doi: 10.1128/iai.9.4.654-657.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. S. Mouse serum factor depressing lymphocyte transformation. Experientia. 1972 Oct 15;28(10):1227–1228. doi: 10.1007/BF01946187. [DOI] [PubMed] [Google Scholar]
- Pavia C. S., Folds J. D., Baseman J. B. Depression of lymphocyte response to concanavalin A in rabbits infected with Treponema pallidum (Nichols strain). Infect Immun. 1976 Jul;14(1):320–322. doi: 10.1128/iai.14.1.320-322.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit J. C., Unanue E. R. Effects of bacterial products on lymphocytes and macrophages: their possible role in natural resistance to listeria infetion in mice. J Immunol. 1974 Sep;113(3):984–992. [PubMed] [Google Scholar]
- Rocklin R. E., MacDermott R. P., Chess L., Schlossman S. F., David J. R. Studies on mediator production by highly purified human T and B lymphocytes. J Exp Med. 1974 Nov 1;140(5):1303–1316. doi: 10.1084/jem.140.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell R. F., Musher D. M. Detection of nonspecific resistance to Listeria monocytogenes in rabbits infected with Treponema pallidum. Infect Immun. 1974 Apr;9(4):658–662. doi: 10.1128/iai.9.4.658-662.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M., Tada T. Dual regulatory role of the thymus in the maturation of immune response in the rabbit. J Exp Med. 1974 Jan 1;139(1):108–127. doi: 10.1084/jem.139.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit B. C., Michael J. G. Immune response suppression by an inhibitor in normal and immune mouse serum. Nat New Biol. 1972 Feb 23;235(60):238–240. doi: 10.1038/newbio235238a0. [DOI] [PubMed] [Google Scholar]
- Wicher V., Wicher K. Cell response in rabbits infected with T. pallidum as measured by the leucocyte migration inhibition test. Br J Vener Dis. 1975 Aug;51(4):240–245. doi: 10.1136/sti.51.4.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicher V., Wicher K. In vitro cell response of Treponema pallidum-infected rabbits. I. Lymphocyte transformation. Clin Exp Immunol. 1977 Sep;29(3):480–486. [PMC free article] [PubMed] [Google Scholar]
- Wicher V., Wicher K. In vitro cell response of Treponema pallidum-infected rabbits. II. Inhibition of lymphocyte response to phytohaemagglutinin by serum of T. pallidum-infected rabbits. Clin Exp Immunol. 1977 Sep;29(3):487–495. [PMC free article] [PubMed] [Google Scholar]



