Abstract
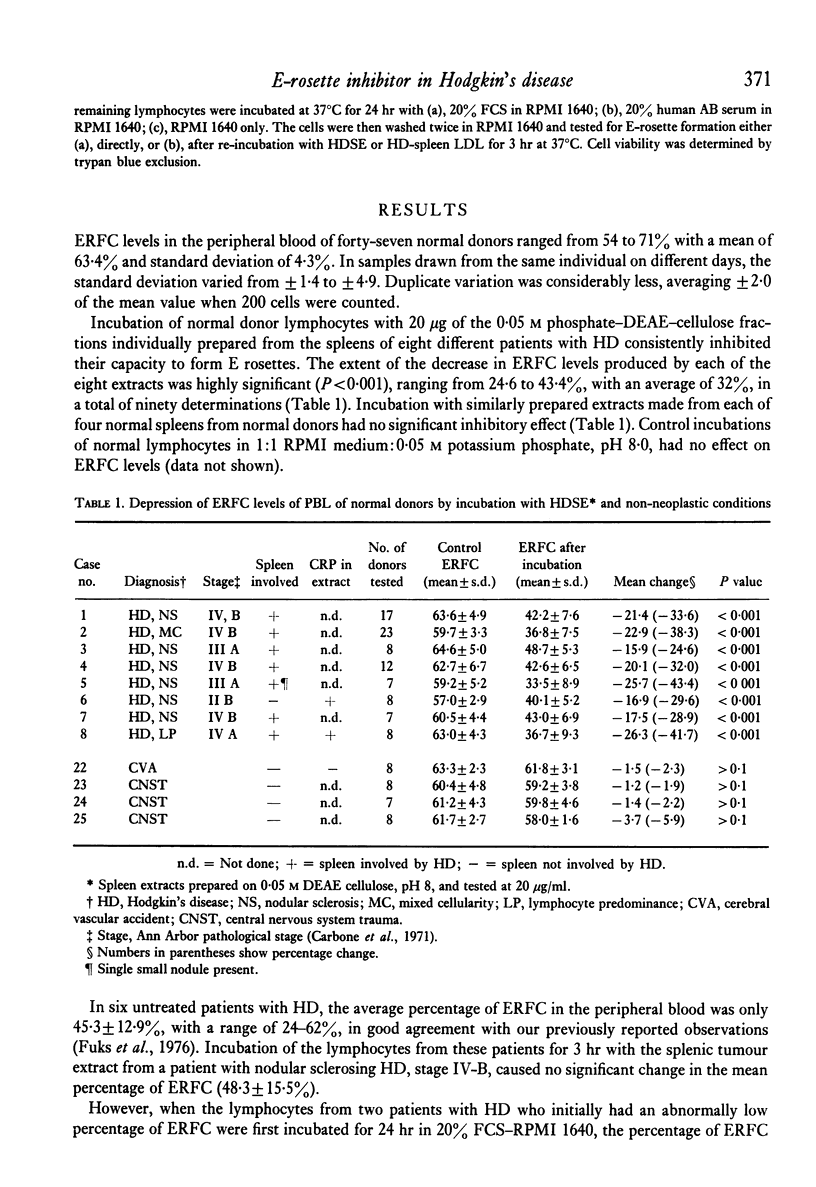
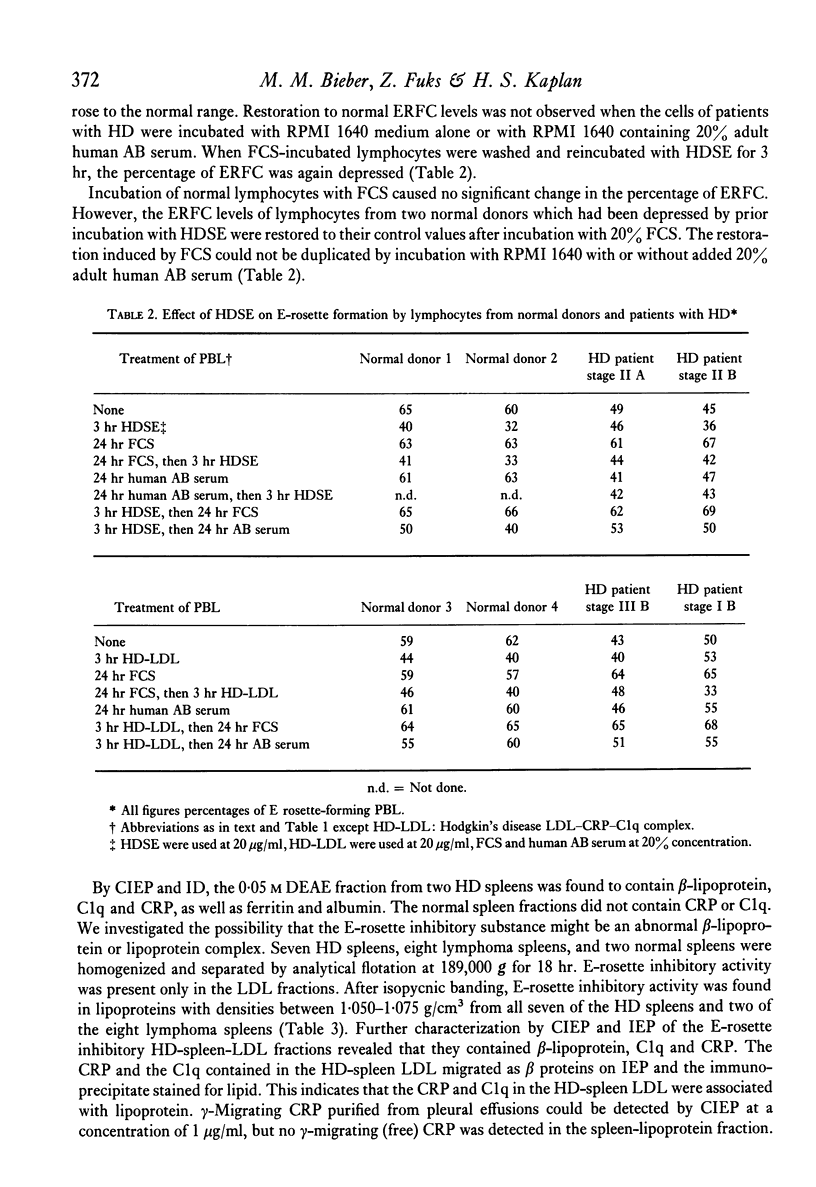
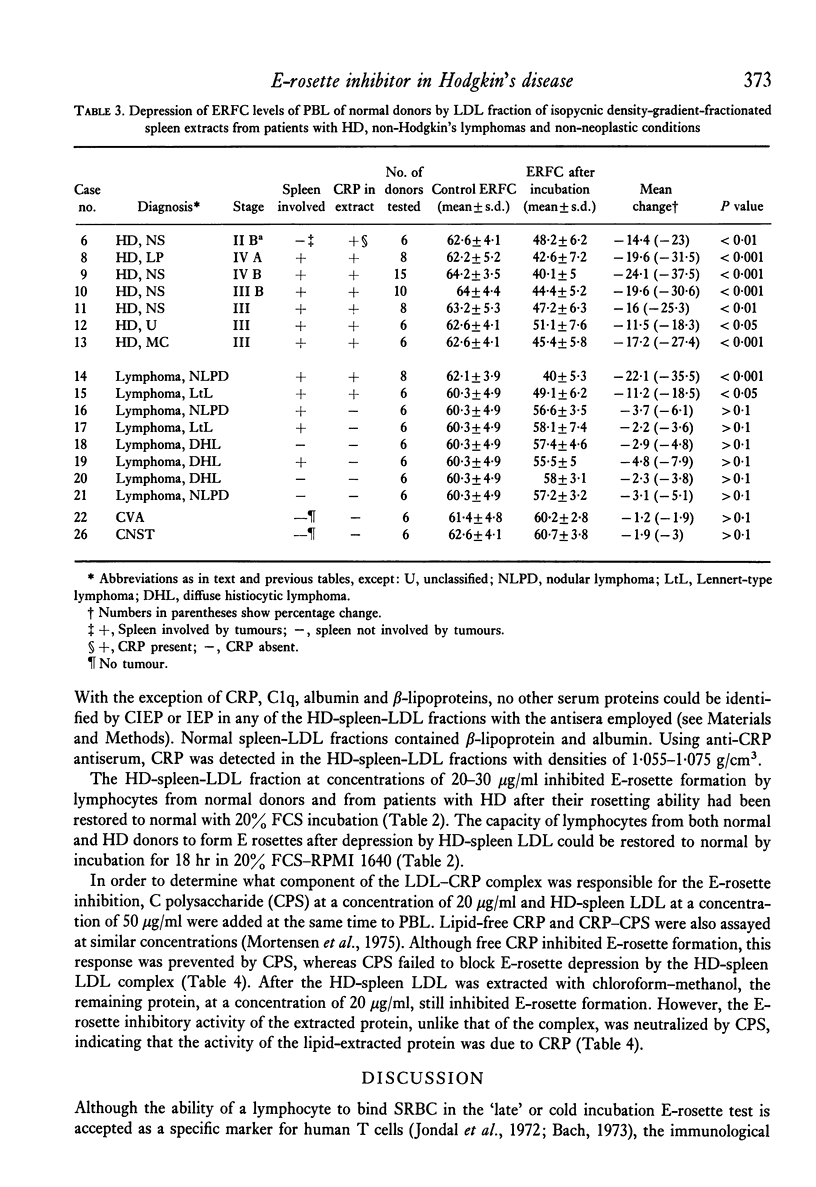
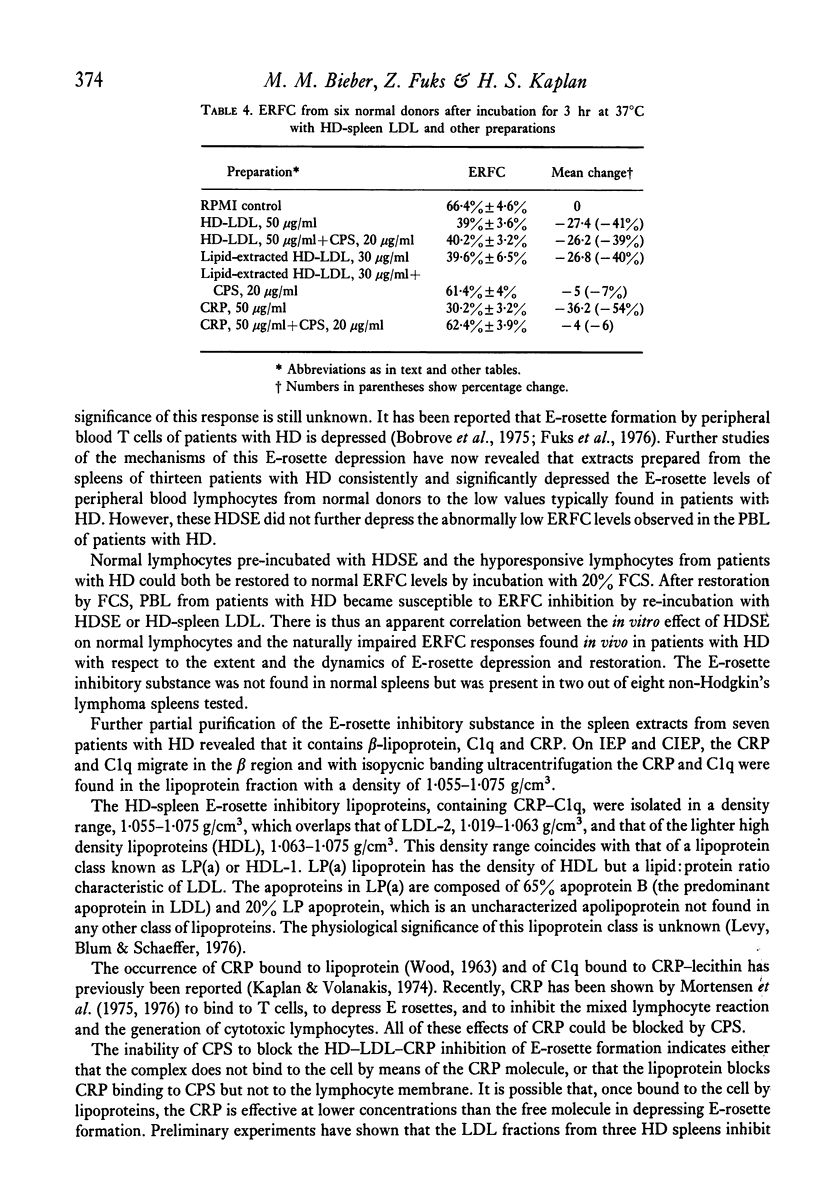
Patients with Hodgkin's disease often manifest impairment of cell-mediated immune responses in both in vivo and in vitro tests, as well as a markedly decreased percentage of E rosette-forming (T) lymphocytes in the peripheral blood. This report describes the inhibition of E-rosette formation by normal peripheral blood lymphocytes after incubation with extracts prepared from the spleens of patients with Hodgkin's disease. Such extracts also depressed E-rosette formation by the peripheral blood lymphocytes of patients with Hodgkin's disease after those cells had been restored to normal function by prior incubation in foetal calf serum. Similarly prepared extracts from the spleens of normal donors had no immunodepressive effect. The E-rosette inhibitory substance in the Hodgkin's disease spleen extracts was found to be a complex containing beta-lipoprotein, C-reactive protein, and C1q.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach J. F. Evaluation of T-cells and thymic serum factors in man using the rosette technique. Transplant Rev. 1973;16(0):196–217. doi: 10.1111/j.1600-065x.1973.tb00121.x. [DOI] [PubMed] [Google Scholar]
- Bieber C. P., Bieber M. M. Detection of ferritin as a circulating tumor-associated antigen in Hodgkin's disease. Natl Cancer Inst Monogr. 1973 May;36:147–157. [PubMed] [Google Scholar]
- Carbone P. P., Kaplan H. S., Musshoff K., Smithers D. W., Tubiana M. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res. 1971 Nov;31(11):1860–1861. [PubMed] [Google Scholar]
- Eltringham J. R., Kaplan H. S. Impaired delayed-hypersensitivity responses in 154 patients with untreated Hodgkin's disease. Natl Cancer Inst Monogr. 1973 May;36:107–115. [PubMed] [Google Scholar]
- Fuks Z., Strober S., King D. P., Kaplan H. S. Reversal of cell surface abnormalities of T lymphocytes in hodgkin's disease after in vitro incubation in fetal sera. J Immunol. 1976 Oct;117(4):1331–1335. [PubMed] [Google Scholar]
- Gaines J. D., Gilmer M. A., Remington J. S. Deficiency of lymphocyte antigen recognition in Hodgkin's disease. Natl Cancer Inst Monogr. 1973 May;36:117–121. [PubMed] [Google Scholar]
- Glatstein E., Guernsey J. M., Rosenberg S. A., Kaplan H. S. The value of laparotomy and splenectomy in the staging of Hodgkin's disease. Cancer. 1969 Oct;24(4):709–718. doi: 10.1002/1097-0142(196910)24:4<709::aid-cncr2820240408>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Goffinet D. R., Glastein E., Kaplan H. S. Herpes zoster infections in lymphoma patients. Natl Cancer Inst Monogr. 1973 May;36:463–464. [PubMed] [Google Scholar]
- Hersh E. M., Oppenheim J. J. Impaired in vitro lymphocyte transformation in Hodgkin's disease. N Engl J Med. 1965 Nov 4;273(19):1006–1012. doi: 10.1056/NEJM196511042731903. [DOI] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMRIN B. B. Successful skin bomografts in mature non-littermate rats treated with fractions containing alpha-globulins. Proc Soc Exp Biol Med. 1959 Jan;100(1):58–61. doi: 10.3181/00379727-100-24522. [DOI] [PubMed] [Google Scholar]
- KELLY W. D., LAMB D. L., VARCO R. L., GOOD R. A. An investigation of Hodgkin's disease with respect to the problem of homotransplantation. Ann N Y Acad Sci. 1960 May 31;87:187–202. doi: 10.1111/j.1749-6632.1960.tb23192.x. [DOI] [PubMed] [Google Scholar]
- Kaplan M. H., Volanakis J. E. Interaction of C-reactive protein complexes with the complement system. I. Consumption of human complement associated with the reaction of C-reactive protein with pneumococcal C-polysaccharide and with the choline phosphatides, lecithin and sphingomyelin. J Immunol. 1974 Jun;112(6):2135–2147. [PubMed] [Google Scholar]
- Khan A., Hill J. M., MacLellan A., Loeb E., Hill N. O., Thaxton S. Improvement in delayed hypersensitivity in Hodgkin's disease with transfer factor: lymphapheresis and cellular immune reactions or normal donors. Cancer. 1975 Jul;36(1):86–89. doi: 10.1002/1097-0142(197507)36:1<86::aid-cncr2820360103>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levy R., Kaplan H. S. Impaired lymphocyte function in untreated Hodgkin's disease. N Engl J Med. 1974 Jan 24;290(4):181–186. doi: 10.1056/NEJM197401242900402. [DOI] [PubMed] [Google Scholar]
- MOWBRAY J. F. Effect of large doses of an alpha2-glycoprotein fraction on the survival of rat skin homografts. Transplantation. 1963 Jan;1:15–20. doi: 10.1097/00007890-196301010-00003. [DOI] [PubMed] [Google Scholar]
- Mannick J. A., Schmid K. Prolongation of allograft survival by an alpha globulin isolated from normal blood. Transplantation. 1967 Jul;5(4 Suppl):1231–1238. doi: 10.1097/00007890-196707001-00063. [DOI] [PubMed] [Google Scholar]
- Mortensen R. F., Gewurz H. Effects of C-reactive protein on the lymphoid system. II. Inhibition of mixed lymphocyte reactivity and generation of cytotoxic lymphocytes. J Immunol. 1976 May;116(5):1244–1250. [PubMed] [Google Scholar]
- Mortensen R. F., Osmand A. P., Gewurz H. Effects on C-reactive protein on the lymphoid system. I. Binding to thymus-dependent lymphocytes and alteration of their functions. J Exp Med. 1975 Apr 1;141(4):821–839. [PMC free article] [PubMed] [Google Scholar]
- Nimberg R. B., Glasgow A. H., Menzoian J. O., Constantian M. B., Cooperband S. R., Mannick J. A., Schmid K. Isolation of an immunosuppressive peptide fraction from the serum of cancer patients. Cancer Res. 1975 Jun;35(6):1489–1494. [PubMed] [Google Scholar]
- Occhino J. C., Glasgow A. H., Cooperband S. R., Mannick J. A., Schmid K. Isolation of an immunosuppressive peptide fraction from human plasma. J Immunol. 1973 Mar;110(3):685–694. [PubMed] [Google Scholar]
- Scheurlen P. G., Schneider W., Pappas A. Inhibition of transformation of normal lymphocytes by plasma factor from patients with Hodgkin's disease and cancer. Lancet. 1971 Dec 4;2(7736):1265–1265. doi: 10.1016/s0140-6736(71)90586-1. [DOI] [PubMed] [Google Scholar]
- WOOD H. F. CRYSTALLIZATION OF C-REACTIVE PROTEIN FOLLOWING REMOVAL OF ASSOCIATED LIPID-CONTAINING MATERIAL BY ANTISERUM TO NORMAL HUMAN BETA LIPOPROTEIN. Yale J Biol Med. 1963 Dec;36:241–248. [PMC free article] [PubMed] [Google Scholar]
- Young R. C., Corder M. P., Haynes H. A., DeVita V. T. Delayed hypersensitivity in Hodgkin's disease. A study of 103 untreated patients. Am J Med. 1972 Jan;52(1):63–72. doi: 10.1016/0002-9343(72)90008-3. [DOI] [PubMed] [Google Scholar]


