Abstract
Intestinal trefoil factor (ITF) is an essential regulator of colonic epithelial restitution, the rapid migration of colonocytes over mucosal wounds. High levels of ITF are frequently present in colorectal cancers and derived cell lines. Mucosal restitution requires the detachment of epithelium from substrate, which would be expected to induce apoptosis. However, mice deficient in ITF showed an increase in colonocyte apoptosis unaccompanied by changes in expression of receptor-related (TNFR/Fas) or stress-related (Bcl-family) cell death regulators. An ITF-expressing colonic (HT-ITF1) cell line was resistant to apoptosis induced by serum starvation and ceramide. Exogenous ITF also protected another human colonic carcinoma-derived cell line (HCT116) and a nontransformed rat intestinal epithelial cell line (IEC-6) from apoptosis. This effect was abrogated by wortmannin and tyrphostin A25, indicating the potential involvement of phosphatidylinositol 3-kinase and epidermal growth factor (EGF) receptor activation. Expression of phosphorylated Akt, which lies downstream of phosphatidylinositol 3-kinase activation, was elevated in this HT-29-ITF line. p53-dependent cell death in the AGS human gastric cancer cell line after etoposide was similarly inhibited by transient expression of ITF but not a C-terminal truncation mutant of ITF, and it required functional phosphatidylinositol 3-kinase and EGF receptor. These findings support a central role for ITF in the maintenance of intestinal mucosal continuity, and conversely demonstrate the potential for ITF expression to confer resistance of colorectal tumors to therapy.
Keywords: cell survival, signal transduction, cell adhesion, colonic neoplasm , cultured tumor cells
Restitution, the ability of epithelial cells to spread and migrate across the basement membrane to cover shallow defects, is the key initial step in repair of mucosal injury and can achieve restoration of mucosal continuity over broad areas of damage within hours (1–3). This resurfacing, which is independent of proliferation, is followed over a period of days by a remodeling phase of epithelial proliferation and differentiation. Brisk restitution after injury is desirable both to limit fluid and electrolyte losses and to prevent diffusion of foreign antigens from the gastrointestinal lumen into local and systemic immune compartments. Both these aspects underlie the morbidity after therapeutic abdominal irradiation, and they limit dose and dose intervals (4).
The trefoil peptide family, comprising the intestinal peptide ITF and the gastric peptides SP and pS2, play a critical role in epithelial restitution within the mammalian gastrointestinal tract. These small (7- 12-kDa) protease-resistant proteins are abundantly secreted onto the mucosal surface by specialized mucus-secreting cells of the gut. They are rapidly up-regulated at the margins of mucosal injury, and they are believed to promote epithelial cell migration (5, 6). Colonic restitution is absent in mice made ITF deficient by homologous recombination (7), rendering otherwise minor colonic injury lethal. Of note, ITF expression is preserved or enhanced in colorectal carcinomas, especially those with a mucinous phenotype (8), which are known to bear an especially poor prognosis, partly because of resistance to conventional chemotherapy and radiotherapy (9, 10).
In common with most epithelial cells, intestinal epithelial cells are anchored; these cells are protected from cell death by maintenance of cell–cell and cell–substratum contacts (11, 12). Detachment normally results in cell death through apoptosis. Since restitution after intestinal epithelial injury involves detachment of cells from these contacts, mechanisms preventing apoptosis of these migrating cells must exist in vivo. We investigated the mechanisms whereby ITF-induced migration might bypass cell death.
Methods
Cell Lines.
HT-29, HCT116, AGS, and IEC-6 cell lines were obtained from the American Type Culture Collection.
Terminal Deoxynucleotidyltransferase-Mediated UTP End Labeling (TUNEL) Assay.
Intestinal tissues from ITF−/− mice of genotype Sv129/C57BL/6 and age-matched Sv129/C57BL/6 and Sv129 purebred controls were fixed in 4% paraformaldehyde/PBS, embedded in paraffin, then sectioned at 4 μm onto coated glass slides. Sections were dewaxed, rehydrated, then briefly digested with 20 μg/ml proteinase K. Apoptotic nuclei were stained by terminal deoxynucleotidyltransferase-catalyzed transfer of fluorescein-labeled nucleotides (Apoptag, Oncor, Gaithersburg, MD), performed according to the manufacturer's instructions. Cells were counterstained with propidium iodide (2.5 μg/ml).
Multiprobe RNase Protection Assay.
Total RNA was extracted as previously described from whole colon mucosa (7) or isolated colonic epithelial cells (13). This RNA was transcribed in vitro in the presence of [α-32P]UTP and proprietary templates as indicated, using the RiboQuant RPA kit (PharMingen), according to the manufacturer's suggested protocol. Briefly, 50 ng of proprietary template was labeled with 100 μCi (1 μCi = 37 kBq) of [α-32P]UTP, using 20 units of T7 RNA polymerase in the presence of 60 μM NTPs, 10 mM DTT, and 40 units of RNasin), then extracted in phenol/chloroform and precipitated with ethanol. Sample RNA (20 μg) or control (2 μg) RNA was hybridized with 1 × 105 cpm probe at 56°C for 16 h, then digested with 20 ng of RNase A for 15 min. Protected bands were resolved on 5% agarose and then autoradiographed.
Plasmids and Transfections.
A full-length human ITF cDNA clone was amplified by PCR from the previously described hITF cDNA (14) by primer extension. Primers were 5′-GGGGTACCGCCGCCACCATGGCTGCCAGAGCGCTCTGCATGCTGGGGCTGGTCCTGGCCTT-3′ and 5′-GCTCTAGAAGGTGCATTCTGCTTCCTG-3′. This product was subcloned into the KpnI/XbaI sites of eukaryote expression vector pcDNA3.1 (Invitrogen). Stable transfection of HT-29 cells with hITF or control pcDNA3.1 plasmids was performed by lipofection using Lipofectamine (GIBCO/BRL) and G418 selection as recommended by the manufacturer. Myc epitope-tagged hITF and C-terminal deletion construct hITF-CMUT were generated by PCR from full-length hITF cDNA and subcloned into pcDNA3.1-myc-hisB (Invitrogen). Primers were 5′-GGTCTAGAGGTACCGCCGCCACCA-3′ and 5′-CCTCTAGAAAGGTGCATTCTGCTT-3′ (hITF-CMYC) and 5′-GGTCTAGAGGTACCGCCGCCACCA-3′ and 5′-CCTCTAGAAAACAACAAGGCACTCC-3′ (hITF-CMUT). Control plasmid pcDNA3.1-myc-his-LacZ was obtained from Invitrogen. AGS cells grown to 50% confluence on plastic chamber slides were transiently transfected by calcium phosphate coprecipitation with 5 μg of pITF-myc, pITF-cmut, p110 [which encodes wild-type phosphatidylinositol 3-kinase (PI 3-kinase)], or pLacZ-myc, with or without 5 μg of dominant-negative pHER653 or Δ-p85 plasmids. Plasmids p110-myc and Δ-p85-myc were kindly provided by R. Xavier (Massachusetts General Hospital). DNA content of transfection mixtures was made constant by the addition of empty vector (pcDNA3.1).
In Vitro Cell Death Assays.
HT-29 wild-type or HT-ITF1 cells were grown to confluence on plastic multiwell plates and incubated with serum-free media where indicated. C2-ceramide (25 μM) was added and cells were incubated for a further 4 h, propidium iodide was added, and cells were photographed by phase-contrast or inverted immunofluorescence microscopy. In studies of other colonic epithelial cell lines, confluent monolayers of HCT116 and IEC-6 colonic epithelial cells in 6-well multiplates were maintained at 0.1% FCS containing medium for 24 h, then further incubated with 1 mg/ml ITF or BSA for 24 h. One millimolar etoposide or 50 mM C2-ceramide was added to HCT116 and IEC-6 cells, respectively, and the cells were incubated for 24 h. For cell death assay after transient transfection, AGS cells were rinsed with PBS 12 h after transfection, then changed to serum-free medium (Ultraculture; BioWhittaker). Twenty-four hours after transient transfection, cells were treated with 50 μM etoposide for 5–6 h, rinsed with PBS, and fixed in 2% paraformaldehyde/PBS containing 0.1% Triton X-100. Myc-expressing cells were labeled with mouse anti-c-myc (Calbiochem) followed by FITC-labeled donkey anti-mouse IgG (Jackson ImmunoResearch). Propidium iodide, 2.5 μg/ml, was added before the final wash. Ten images at ×100 magnification were captured by confocal immunofluorescence microscopy, and at least 100 transfected cells were scored for having apoptotic morphology. Results shown are representative of three independent experiments.
Western Blot Analysis for ITF.
Cells were washed with PBS and immediately lysed on ice in buffer (1% Triton X-100/50 mM Tris⋅HCl, pH 7.4/150 mM NaCl containing 1 mM EDTA, 10 μg/ml aprotinin, 100 μM phenylmethylsulfonyl fluoride, and 10 μg/ml leupeptin) containing phosphatase inhibitors (200 μM sodium orthovanadate, 100 mM NaF, and 10 mM sodium pyrophosphate). The lysates were centrifuged at 10,000 × g for 20 min at 4°C, and the protein concentration in resulting supernatants was measured by the Bradford method (Bio-Rad). Samples were electrophoresed through 15–18% polyacrylamide gel and transferred onto poly(vinylidene difluoride) (PDVF) membranes (Millipore). After saturation in blocking buffer (3% BSA in 0.05% Tween 20), blots were incubated for 16 h at 4°C with polyclonal antiserum from rabbits immunized with recombinant rat ITF peptide. Renaissance chemiluminescent reagents (DuPont) were used according to the manufacturer's instructions.
Western Blotting for Poly(ADP-ribose) Polymerase (PARP).
PARP cleavage was assessed after induction of apoptosis in HT-vec, HT-ITF1 cells, and parental HT-29 cells as well as HCT116 and IEC-6 cells. Cells were collected into buffer [62.5 mM Tris⋅HCl, pH 6.8/6 M urea/10% (vol/vol) glycerol/2% (vol/vol) SDS/0.00125% bromophenol blue/5% (vol/vol) 2-mercaptoethanol], sonicated for 15 sec, then denatured at 65°C for 15 min. Samples were run on SDS/PAGE gels (4–12% Bis-Tris gel; NOVEX, San Diego) and transferred to nitrocellulose filters. The transfers were then incubated for 16 h with anti-PARP (Biomol Research Laboratories, Plymouth Meeting, PA) diluted 1:1000 in the blocking buffer. The blots were washed twice in TBS-T (50 mM Tris⋅HCl, pH 7.4/0.15 M NaCl/0.1% Tween 20), then incubated for 1 h with horseradish peroxidase-conjugated donkey anti-rabbit IgG (Amersham Life Science) diluted 1:2000.
Results
ITF−/− Mice Have Increased Intestinal Apoptosis.
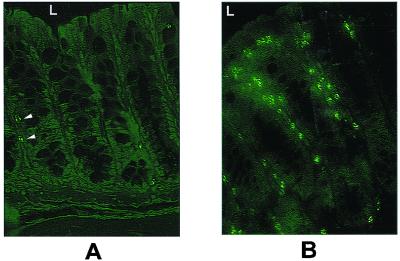
In previous studies, an increase in BrdU labeling of proliferating cells in the colon of adult ITF-null mice was noted (7). However, cell number per colonic crypt appeared normal, suggesting that an increase in cell loss may underlie the drive to proliferation. The majority of physiological colonocyte losses occur through apoptosis (15). Accordingly, we assessed colonic apoptosis by TUNEL staining of colonic sections fixed with paraformaldehyde and embedded in paraffin. As it was formally possible that purebred Sv129 mice may have had a higher rate of spontaneous colonic apoptosis, and to control for possible differences in Sv129 contribution to the ITF-null genotype, both Sv129/C57BL/6 wild-type mice and purebred Sv129 mice were assessed for control purposes. In both control strains, TUNEL-positive cells occurred at a frequency of less than one per colonic crypt (Fig. 1), and these cells also had morphological hallmarks of apoptosis. An increase in the number of TUNEL-positive cells was found in the colonic crypts of ITF-null mice (Fig. 1). These TUNEL-positive cells were distributed along the height of the crypts and into the surface epithelial cuff, rather than the expected location near the stem cell compartment (4). Similar findings were made in small intestinal crypt–villus units (data not shown).
Figure 1.
Colonic epithelial cell apoptosis is increased in ITF-deficient mice. TUNEL labeling of apoptotic nuclei was performed on matched 4-μm sections of distal colon from 6-week-old ITF-null mice (n = 15) and age-matched wild-type Sv129/C57/Bl6 mice (n = 15). (A) Apoptotic nuclei of wild-type distal colon. Two to three scattered positive nuclei per crypt are seen (arrowheads), corresponding to the frequency of apoptotic cells seen with conventional light microscopy. L indicates lumen, for orientation. (B) Section of distal colon from ITF-null mouse, showing an increase in labeled cells from the mid-crypt to the surface cuff. Note that positive nuclei lie immediately basal to the crypt epithelium. Features shown are representative of studies performed in 15 paired ITF-null mice and controls and 5 purebred Sv129 mice.
Agents use to induce intestinal apoptosis in mouse models typically result in preferential death of cells in the stem cell positions or the adjacent compartment, which is thought to represent selection of cells according to replication phase (4). The broad distribution of cell death seen in ITF−/− crypts, in the presence of normal gland architecture and cell repertoire, suggests that sensitivity to apoptosis was occurring in colonocytes at differing stages of proliferation and differentiation, and this suggests the influence of local triggers to cell death.
ITF−/− Mice Have Normal Expression of bcl-2 Family and Tumor Necrosis Factor (TNF) Receptor Superfamily Genes.
ITF is an immediate-early gene able to regulate the expression of the gastric trefoil genes SP and pS2 as well as other immediate-early genes in model cell lines (16). Accordingly, potential regulation by ITF of genes critical in apoptosis was evaluated. For example, whereas colonic epithelial apoptosis occurs throughout the crypt, homozygous bcl-2-null mice have increased colonic apoptosis selective for the crypt base (17). In addition, normal colonocyte death may be mediated by CD95 (Fas); indeed, Fas expression is attenuated or lost in colon carcinoma (18). Apoptosis mediated by Fas/Fas ligand may also be regulated by the level of Fas ligand, TNF receptor superfamily, or bcl-2 family mRNA expression (19, 20, 21).
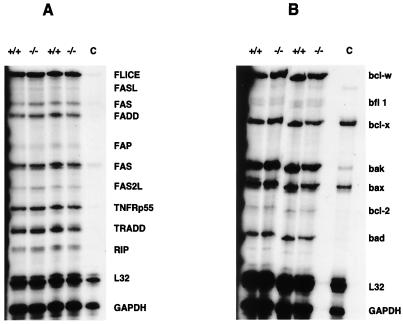
This possibility was tested by multiple-probe RNase protection assays of total RNA extracted from the full thickness of the mouse colon, or RNA from isolated mouse colonic crypt epithelial cells (13). No differences in gene expression of any proteins related to the receptor-mediated apoptotic pathway, including Fas/Fas ligand, FADD/Mort-1, FLICE, and TNF receptor superfamily members could be detected (Fig. 2A). Similarly, there were no differences in gene expression of stress-related apoptosis proteins, including bcl-2 or Bak/Bcl-x/Bad (Fig. 2B).
Figure 2.
Increase in colonic apoptosis is not associated with transcriptional regulation of bcl-2 family or TNF receptor family regulatory proteins. Total RNA was isolated from whole colon mucosa (not shown) or from isolated epithelial cells (19), as shown, from two representative adult wild-type (+/+) and ITF-null (−/−) mice each and analyzed by multiprobe RNase protection assay. Lane C contained mouse control RNA. (A) Expression of Fas, Fas ligand, and related surface membrane-associated mediators of apoptosis. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (B) Expression of bcl-2 and related modifiers of apoptosis.
ITF Activates PKB/Akt Kinase and Prevents p53-Independent Apoptosis in Colon Cancer and Nontransformed Intestinal Epithelial Cell Lines.
These data indicated that ITF might act locally at the posttranslational level to protect intestinal epithelial cells against cell death. This suggestion contrasts with a recent report of Efstathiou et al. (22), where the addition of bacterially expressed ITF led to detachment and death of HT-29 cells. This hypothesis was tested by in vitro analysis of apoptosis induced by various agents.
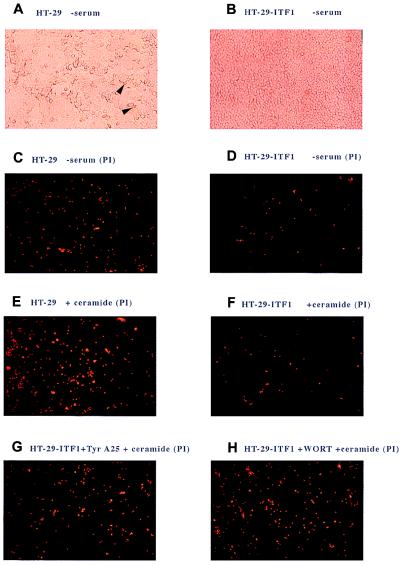
Both p53-dependent and -independent mechanisms contribute to intestinal cell death (15). We studied p53-independent apoptosis in HT-29 colon cancer cells, which lack functional p53 and are therefore relatively insensitive to p53-mediated apoptosis (23, 24); conveniently, these cells also lack ITF expression (14). HT-29 cells stably transfected with an expression plasmid encoding full-length ITF (HT-ITF1) or vector control (HT-vec) were grown as confluent monolayers on plastic. Expression of ITF by the HT-ITF1 line was confirmed by Western blot analysis. In contrast, the vector control parental line produced no detectable ITF. Vector control cells were relatively sensitive to apoptosis induced by 18 h of serum withdrawal (Fig. 3 A and C). In contrast, HT-ITF1 cells were resistant to serum withdrawal, with a reduction by up to 75% in the number of cells undergoing apoptosis (Fig. 3 B and D). Comparable results were obtained from four independently derived ITF-expressing clones (data not shown). HT-vec and HT-ITF1 cells were then treated with C2-ceramide for 3 h. This treatment caused a 4-fold rise in the number of apoptotic HT-vec cells (Fig. 3E) but had no appreciable effect on HT-ITF1 cells (Fig. 3F). Protection from cell death conferred by ITF expression occurred in the presence or absence of serum, indicating that ITF may act independently of serum growth factors.
Figure 3.
Morphological features of apoptosis in parental and ITF-expressing HT-29 cells after serum starvation and ceramide-induced apoptosis. (A) Phase-contrast view of parental HT-29 colon cancer cells, seeded at 1 × 106 cells per 2-cm well and grown to confluence, followed by 24 h of serum withdrawal. Detaching cells with cytoplasmic vacuolation are indicated by arrows. (B) Phase-contrast view of HT-ITF1 cells. After serum withdrawal as in A, comparable numbers of total cells are present relative to parental cells in A. (C) Dark-field view of confluent parental HT-29 cells after serum withdrawal, as in A; apoptotic nuclei counterstained with propidium iodide (PI). (D) Dark-field view, HT-ITF1 cells, serum withdrawal. (E) Parental HT-29 cells, 3 h after addition of C2 ceramide (25 μM). (F) HT-ITF1 cells 3 h after C2 ceramide. (G) HT-ITF1 cells pretreated with tyrphostin A25 (30 μM), 30 min before addition of C2 ceramide. (H) HT-ITF1 cells pretreated with wortmannin (100 nM), 30 min before C2 ceramide.
Although receptors for trefoil peptides have not been cloned, ITF leads to phosphorylation of the epidermal growth factor (EGF) receptor (EGFR) (25), which is necessary for the mitogen-activated protein kinase (MAPK)-dependent transcriptional cross-regulation exhibited by trefoil peptides (16). The EGFR, while stimulating classical mitogenic pathways in epithelial cells, also mediates the association of β-catenin with the actin cytoskeleton and components of the intracellular adherens junction (26, 27). The EGFR therefore is central to a variety of epithelial cell properties, from growth and wound healing to adhesion and cell migration. Colonocyte cell death induced by loss of cell–cell and cell–substratum contacts in vitro can be prevented by EGF as well as scatter factor/HGF (12). Protection from detachment-induced apoptosis appears to require the activity of PI 3-kinase and consequent activation of PKB/Akt (28, 29). We therefore determined whether the ability of ITF to protect from cell death induced by serum withdrawal or C2-ceramide might require EGFR activation or the activity of PI 3-kinase. Inhibition of EGFR tyrosine kinase activity by pretreatment of cells with the inhibitor tyrphostin A25 (30, 31) restored sensitivity of HT-ITF1 cells to both serum withdrawal (data not shown) and C2-ceramide (Fig. 3G). Similarly, pretreatment with the PI 3-kinase inhibitor wortmannin rendered HT-ITF1 cells sensitive to C2-ceramide (Fig. 3H).
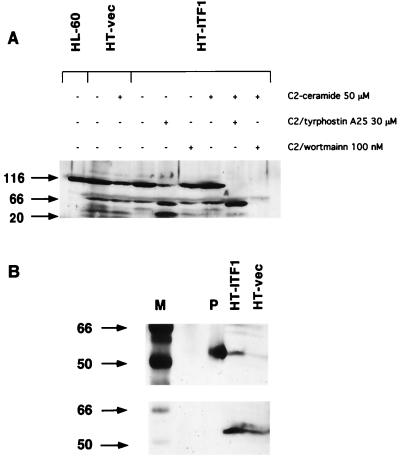
Sensitivity of HT-ITF1 cells to ceramide and serum starvation was also assessed by cleavage of the p116 caspase substrate PARP. Treatment of HT-vec but not HT-ITF1 cells with C2-ceramide at 50 μM was associated with cleavage of p116 PARP (Fig. 4A, lanes 2 and 3); HT-ITF1 cells were resistant to C2 ceramide-induced PARP cleavage. Treatment with tyrphostin was associated with PARP cleavage and the appearance of the p85 and p20.1 fragments both in the absence (Fig. 4A, lane 5) and in the presence (Fig. 4A, lane 8) of C2-ceramide. Wortmannin pretreatment was associated with loss of p116 after C2-ceramide treatment (Fig. 4A, lane 9), but not in the absence of ceramide, in HT-ITF1 cells.
Figure 4.
Expression of ITF in colon cancer cells leads to PI 3-kinase and EGFR-dependent resistance to cell death. (A) Resistance to ceramide-induced PARP cleavage in HT-ITF1 cells. HT-vec or HT-ITF1 cells were grown to confluence and lysed 24 h after the addition of ceramide. The inhibitors tyrphostin A25 and wortmannin were added 30 min before ceramide where indicated. Serum starvation was associated with the appearance of p20.1 fragment of HT-vec but not HT-ITF1 cells (lanes 2 and 4). Loss of p116 PARP in vector control cells after ceramide (lane 3) did not occur in HT-ITF1 cells (lane 7). Pretreatment of HT-ITF1 cells with tyrphostin was associated with loss of p116 after serum starvation (lane 5) and total loss of p116 after ceramide (lane 8), with predominant p85 and p20.1 bands. Loss of p116 also occurred in HT-ITF1 cells treated with wortmannin and ceramide (lane 9). Equal protein loading in all lanes was assessed by Coomassie staining. HL-60: control lysate from HL-60 cells (lane 1). (B) Increased phosphorylation of PKB/Akt in HT-ITF1 cells. Lysates from serum-stimulated HT-ITF1 or vector control HT-vec cells were separated on SDS/PAGE and immunoblotted with phospho-specific Akt antibody (Upper), then reblotted with total Akt antibody (Lower). M, molecular mass markers (kDa); P, positive control (phosphorylated Akt).
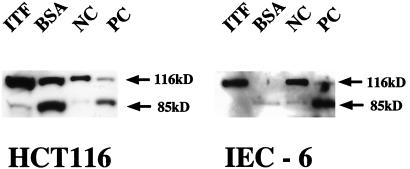
Although ITF has been reported to induce EGFR phosphorylation in colon cancer cells (23), involvement of PI 3-kinase-dependent pathways was unexpected. The antiapoptotic action of PI 3-kinase activation requires activation of PKB/Akt and PKB/Akt-dependent phosphorylation of the bcl-2 family member BAD (32, 33). Accordingly, we assessed PKB/Akt activation in serum-stimulated HT-29 cells by using antibody specifically recognizing the Ser-457-phosphorylated form of PKB/Akt. Indeed, serine phosphorylation of PKB/Akt was found to be increased in HT-ITF1 cells compared with HT-vec (Fig. 4B). The effects of trefoil peptide in blocking induced apoptosis were not unique to the HT-29 cell line. As demonstrated in Fig. 5, addition of ITF blocked p53-dependent apoptosis induced by etoposide in the HCT116 cells, another human colon cancer-derived cell line that does not express endogenous trefoil. Moreover, ITF also prevented etoposide-induced apoptosis in a nontransformed rat intestinal epithelial cell line (IEC-6). Thus, significant cleavage of full-length PARP (116 kDa) was observed in these lines after treatment with etoposide or C2-ceramide in the presence of the control protein (BSA). In contrast, PARP cleavage was almost completely prevented by addition of ITF.
Figure 5.
Western blot analysis of PARP cleavage of HCT116 and IEC-6 cells. After 24 h of preincubation with 1 mg/ml ITF or BSA, apoptosis was induced by addition of 1 mM etoposide or 50 mM C2-ceramide to the cultures. Total cell lysates were subjected to Western blotting with anti-PARP. ITF-treated HCT116 and IEC-6 cells were protected from apoptosis. NC and PC represent apoptosis-noninduced and apoptosis-induced controls of human HL60 leukemia cell, respectively.
ITF Prevents p53-Dependent Apoptosis in Vitro in an EGFR- and PI 3-Kinase-Dependent Manner.
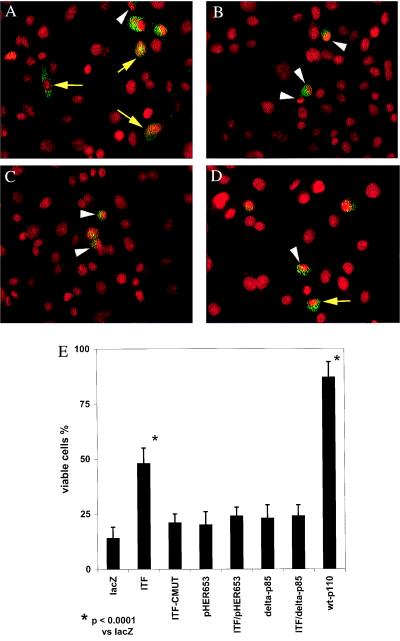
After studies using the p53-deficient HT-29 clones, we explored the effect of ITF on p53-dependent apoptosis. The cancer chemotherapeutic agent etoposide induces apoptosis through p53-dependent mechanisms (34). In contrast to HT-29 cells, which are insensitive to etoposide because they lack p53, cells of the gastric cancer line AGS are sensitive to etoposide-induced apoptosis, show minimal ITF expression, and are amenable to transient transfection (16). We therefore transiently transfected AGS cells with epitope-tagged ITF (ITF-Myc), mutant ITF (ITF-CMUT), or control (LacZ) expression plasmids. Apoptosis was then induced by the addition of etoposide to 50 μM in complete medium, and cells were incubated for a further 5–6 h to allow nuclear changes to occur. Apoptosis in transfected cells was assessed by nuclear morphology of Myc-expressing cells counterstained with propidium iodide. Of control (LacZ-Myc) transfected cells, 85.5% showed characteristic nuclear morphology of apoptosis, including intense propidium iodide staining within a condensed, scalloped, or fragmented nucleus. ITF-expressing cells generally appeared viable, with 48% of cells exhibiting normal nuclear morphology (Fig. 6A). This degree of protection was statistically significant (P = 0.0001; χ2). No protection was seen after expression of a mutant ITF lacking the C terminus of the protein but possessing the N terminus and complete trefoil domain (Fig. 6B), with 81% of cells showing nuclear changes of apoptosis (P = 0.033 compared with wild-type ITF; χ2 with continuity correction).
Figure 6.
ITF protects against p53-dependent apoptosis in a PI 3-kinase- and EGFR-dependent manner. AGS cells were transiently transfected with the indicated constructs and apoptosis was induced by addition of 50 μM etoposide. (A) ITF-expressing cells (green punctate cytoplasmic staining), showing cells of normal nuclear morphology (yellow arrows) and cells with apoptotic nuclear morphology (white arrowhead). (B) Cells expressing a C-terminally truncated ITF mutant protein, showing apoptotic morphology with condensed and scalloped nucleus (white arrowheads). (C) AGS cells cotransfected with wild-type ITF and pHER653. White arrowheads indicate apoptotic cells. (D) AGS cells expressing wild-type ITF in the presence of Δ-p85. (E) Summary of results after transfection of AGS cells with the indicated constructs and treatment with etoposide; wt-p110: wild-type PI 3-kinase.
Subsequently, AGS cells were cotransfected with ITF-Myc and pHER653, a construct encoding a dominant-negative (kinase domain deleted) EGFR, before induction of apoptosis with etoposide (Fig. 6C). This treatment reduced the number of viable cells from 47.4% to 22.9% (P = 0.02; χ2), confirming that EGFR activation contributes to antiapoptotic signaling in ITF-expressing cells. This was similar to the number of viable cells seen after transfection with pHER653 in the absence of ITF (Fig. 6E). Transfection with an expression plasmid encoding the wild-type catalytic subunit of PI 3-kinase before initiation of apoptosis (p110, Fig. 6E) was protective, with 88% of transfected cells showing normal nuclear morphology. Cotransfection of AGS cells with ITF-Myc and dominant-negative PI 3-kinase (delta-p85, lacking the binding site for p110) prevented ITF-mediated protection against etoposide-induced apoptosis (Fig. 6 D and E).
Discussion
ITF is a member of the trefoil peptide family normally expressed throughout the small and large intestine by goblet epithelial cells. Of note, many colon cancers and their derivative cell lines are marked by preserved ever-enhanced ITF production. In the present studies, we demonstrate that both endogenous and exogenous ITF may prevent p53-dependent and p53-independent apoptosis. This effect was observed in multiple intestinal cell lines, including both cancer-derived malignant and nontransformed cells. These results suggest a twofold role for ITF in the intestine. Where mucosal continuity is maintained the presence of the protease-resistant ITF may prevent inappropriate activation of cell death pathways in the presence of trivial stresses. Where mucosal breaches have occurred, the marked up-regulation of trefoil peptides in the wound margin may provide protection for the monolayer of epithelial cells that must migrate across the defect. Nevertheless, short-term (3–4 days) losses of large areas of intestinal epithelium may arise in the small intestine after abdominal irradiation (4) and in the colon after infectious and idiopathic colitis, and may overwhelm the capacity for regeneration. This observation suggests a therapeutic use for ITF in this setting. Trefoil peptides are highly protease resistant and active after oral (35) or rectal (7) administration. Rectal instillation of ITF at 12-h intervals was able to rescue the colonic mucosa of ITF-null mice to wild type and to prevent the marked ulceration seen after oral administration of dextran sulfate sodium (7).
The posttranslational action of ITF to protect intestinal epithelial cells against apoptosis appears to contradict a preliminary report in which bacterially expressed ITF led to detachment and death of HT-29 cells (22). Several lines of evidence favor ITF having such a protective role, including in vivo evidence of increased colonocyte proliferation in ITF-null mice (7) and increased spontaneous colonic apoptoses in these mice. In addition, ITF stimulation of HT-29 and other gastrointestinally derived cell lines leads to phosphorylation of the EGFR (25) and EGFR-dependent activation of mitogen-activated protein kinase (16), which would be expected to favor survival rather than apoptosis. Furthermore, activation of the kinase Akt has also been implicated in survival mechanisms of a range of cell types (32, 33), and it occurs in cells stably expressing ITF (this report), and in other gastrointestinal cell lines stimulated with recombinant ITF or SP (D.R.T. and D.K.P., unpublished observations). The functional importance of Akt phosphorylation was underlined by the abrogation of the protective effects of ITF in the presence of PI 3-kinase inhibitors.
What are the proximal signaling events after ITF stimulation suggested by these data? The kinetics of EGFR phosphorylation after EGF and ITF stimulation are similar (16). However, direct binding of ITF to the EGFR has not been established (36). In addition, the specific erbB receptor dimers activated after ITF stimulation are not established. Transactivation of distinct erbB homodimers and heterodimers appears to dictate subsequent signaling events, including receptor internalization and recruitment of adapter proteins, and the kinetics of the association between the EGFR and the p85 subunit of PI 3-kinase (37). Although it appears that EGFR–p85 interaction is critical for inhibition of apoptosis achieved by ITF treatment, these issues are the focus of biochemical studies directed toward the molecular ordering of the ITF-stimulated state.
Expression of ITF rendered cancer cell lines resistant to induction of apoptosis by a variety of agents. Does ITF expression result in a more aggressive colon cancer phenotype? This may not be the case: expression of ITF in a panel of resected colon cancers was associated with more differentiated and less advanced tumors (8). However, it is possible that ITF may confer invasive potential and resistance to therapy. Mucinous or colloidal colorectal cancers, which express abundant ITF (8), have a particularly invasive character despite moderate degrees of differentiation; furthermore, these tumors are frequently resistant to chemotherapy and radiotherapy (9, 10). It follows then that the use of in vitro/in vivo models of tumor invasion and metastasis to determine the place of ITF in cancer progression is a priority.
Acknowledgments
We thank Dr. M. Korc for the plasmid RK5-HER653, Dr. R. Xavier for the plasmids p110-myc and Δ-p85-myc, Dr. S. Brandwein for technical assistance, Drs. D. Brown and R. Nishiyama for technical advice, and Drs. D. L. Vaux and T. C. Wang for many helpful discussions. This work was supported by National Institutes of Health Grants DK 43351 and DK 46906 and a Postdoctoral Fellowship from the Crohn's and Colitis Foundation of America.
Abbreviations
- ITF
intestinal trefoil factor
- EGF
epidermal growth factor
- EGFR
EGF receptor
- PI 3-kinase
phosphatidylinositol 3-kinase
- PARP
poly(ADP-ribose) polymerase
- TUNEL
terminal deoxynucleotidyltransferase-mediated UTP end labeling
- TNF
tumor necrosis factor
References
- 1.Nusrat A, Delp C, Madara J L. J Clin Invest. 1992;89:1501–1511. doi: 10.1172/JCI115741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santos M F, McCormack S A, Guo Z, Okolicany J, Zheng Y, Johnson L R, Tigyi G. J Clin Invest. 1997;100:216–225. doi: 10.1172/JCI119515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dieckgraefe B K, Stenson W F, Alpers D H. Curr Opin Gastroenterol. 1996;12:109–114. [Google Scholar]
- 4.Potten C S, Merritt A, Hickman J, Hall P, Faranda A. Int J Radiat Biol. 1994;65:71–78. doi: 10.1080/09553009414550101. [DOI] [PubMed] [Google Scholar]
- 5.Dignass A, Lynch-Devaney K, Kindon H, Thim L, Podolsky D K. J Clin Invest. 1994;94:376–383. doi: 10.1172/JCI117332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sands B E, Podolsky D K. Annu Rev Physiol. 1996;58:253–273. doi: 10.1146/annurev.ph.58.030196.001345. [DOI] [PubMed] [Google Scholar]
- 7.Mashimo H, Wu D-C, Podolsky D K, Fishman M C. Science. 1996;274:262–265. doi: 10.1126/science.274.5285.262. [DOI] [PubMed] [Google Scholar]
- 8.Taupin D R, Ooi K, Yeomans N D, Giraud A S. Lab Invest. 1996;75:25–32. [PubMed] [Google Scholar]
- 9.Lev R, Lee M. In: Gastrointestinal Cancers. Rustgi A K, editor. Philadelphia: Lippincott-Raven; 1995. pp. 379–398. [Google Scholar]
- 10.Bresalier R S, Ho S B, Schoeppner H L, Kim Y S, Sleisenger M H, Brodt P, Byrd J C. Gastroenterology. 1996;110:1354–1367. doi: 10.1053/gast.1996.v110.pm8613039. [DOI] [PubMed] [Google Scholar]
- 11.Frisch S M, Francis H. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hague A, Hicks D J, Bracey T S, Paraskeva C. Br J Cancer. 1997;75:960–968. doi: 10.1038/bjc.1997.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meijssen M A, Brandwein S L, Reinecker H C, Bhan A K, Podolsky D K. Am J Physiol. 1998;274:G472–G479. doi: 10.1152/ajpgi.1998.274.3.G472. [DOI] [PubMed] [Google Scholar]
- 14.Podolsky D K, Lynch-Devaney K, Stow J L, Oates P, Murgue B, deBeaumont M, Sands B E, Mahida Y R. J Biol Chem. 1993;268:6694–6702. [PubMed] [Google Scholar]
- 15.Potten C S. Am J Physiol. 1997;273:G253–G257. doi: 10.1152/ajpgi.1997.273.2.G253. [DOI] [PubMed] [Google Scholar]
- 16.Taupin D, Wu D C, Jeon W K, Devaney K, Wang T C, Podolsky D K. J Clin Invest. 1999;103:R31–R38. doi: 10.1172/JCI3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merritt A J, Potten C S, Watson A J, Loh D Y, Nakayama K, Nakayama K, Hickman J A. J Cell Sci. 1995;108:2261–2271. doi: 10.1242/jcs.108.6.2261. [DOI] [PubMed] [Google Scholar]
- 18.von Reyher U, Stråter J, Kittstein W, Gschwendt M, Krammer P H, Moller P. Cancer Res. 1998;58:526–534. [PubMed] [Google Scholar]
- 19.Bissonnette R P, Brunner T, Lazarchik S B, Yoo N J, Boehm M F, Green D R, Heyman R A. Mol Cell Biol. 1995;15:5576–5585. doi: 10.1128/mcb.15.10.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su X, Cheng J, Liu W, Liu C, Wang Z, Yang P, Zhou T, Mountz J. J Immunol. 1998;160:5288–5293. [PubMed] [Google Scholar]
- 21.Messineo C, Jamerson M H, Hunter E, Braziel R, Bagg A, Irving S G, Cossman J. Blood. 1998;91:2443–2451. [PubMed] [Google Scholar]
- 22.Efstathiou J A, Noda M, Rowan A, Dixon C, Chinery R, Jawhari A, Hattori T, Wright N A, Bodmer W F, Pignatelli M. Proc Natl Acad Sci USA. 1998;95:3122–3127. doi: 10.1073/pnas.95.6.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodrigues N R, Rowan A, Smith M E, Kerr I B, Bodmer W F, Gannon J V, Lane D P. Proc Natl Acad Sci USA. 1990;87:7555–7559. doi: 10.1073/pnas.87.19.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ossina N K, Cannas A, Powers V C, Fitzpatrick P A, Knight J D, Gilbert J R, Shekhtman E M, Tomei L D, Umansky S R, Kiefer M C. J Biol Chem. 1997;272:16351–16357. doi: 10.1074/jbc.272.26.16351. [DOI] [PubMed] [Google Scholar]
- 25.Liu D, el-Hariry I, Karayiannakis A J, Wilding J, Chinery R, Kmiot W, McCrea P D, Gullick W J, Pignatelli M. Lab Invest. 1997;77:557–563. [PubMed] [Google Scholar]
- 26.Hoschuetzky H, Aberle H, Kemler R. J Cell Biol. 1994;127:1375–1380. doi: 10.1083/jcb.127.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi K, Suzuki K, Tsukatani Y. Oncogene. 1997;15:71–78. doi: 10.1038/sj.onc.1201160. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Viciana P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 29.Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne P H, Downward J. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lyall R M, Zilberstein A, Gazit A, Gilon C, Levitzki A, Schlessinger J. J Biol Chem. 1989;264:14503–14509. [PubMed] [Google Scholar]
- 31.Levitzki A, Gazit A. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- 32.del Peso L, González-García M, Page C, Herrera R, Nuñez G. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 33.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 34.Lowe S W, Ruley H E, Jacks T, Houseman D E. Cell. 1993;74:957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- 35.Babyatsky M W, deBeaumont M, Thim L, Podolsky D K. Gastroenterology. 1996;110:489–497. doi: 10.1053/gast.1996.v110.pm8566596. [DOI] [PubMed] [Google Scholar]
- 36.Otto W R, Rao J, Cox H M, Kotzian E, Lee C Y, Goodlad R A, Lane A, Gorman M, Freemont P A. Eur J Biochem. 1996;235:64–72. doi: 10.1111/j.1432-1033.1996.00064.x. [DOI] [PubMed] [Google Scholar]
- 37.Olayioye M A, Graus-Porta D, Beerli R R, Rohrer J, Gay B, Hynes N E. Mol Cell Biol. 1998;18:5042–5051. doi: 10.1128/mcb.18.9.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]