Abstract
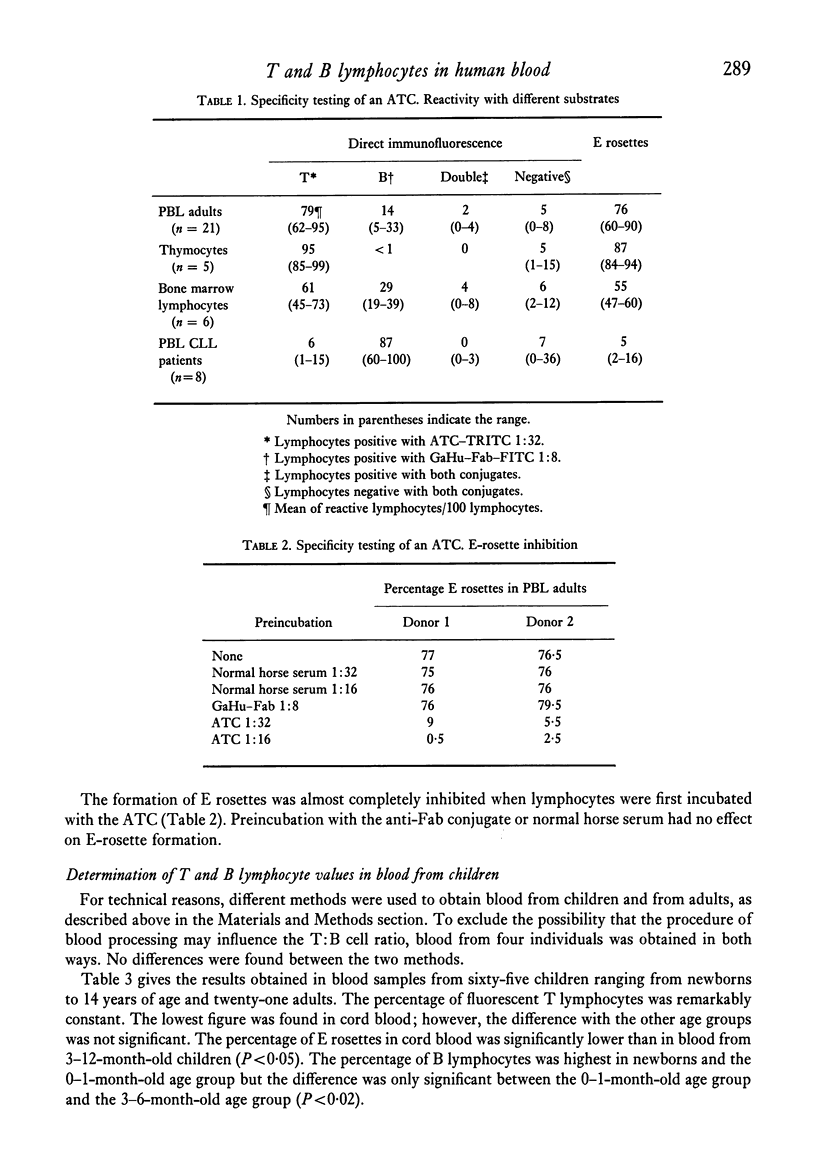
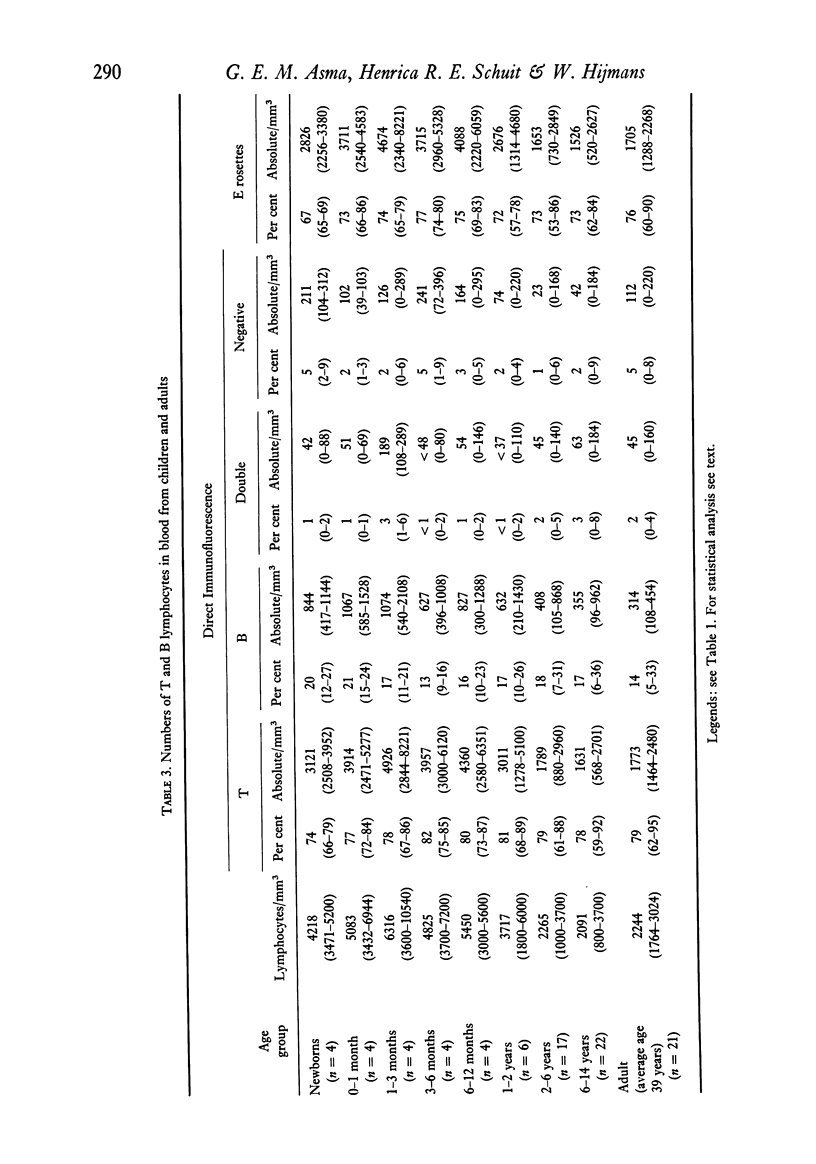
Lymphocytes in the peripheral blood of sixty-five children ranging in age from newborns to 14-year-olds and twenty-one adults were studied by the direct immunofluorescence technique for B- and T-membrane determinants, with a GaHu–Fab fluorescein isothiocyanate (FITC) conjugate as a B-cell marker and a tetramethyl rhodamine isothocyanate (TRITC) labelled horse anti-human T-cell conjugate (ATC) as a T-cell marker. The ATC was prepared from a commercial horse anti-human thymocyte IgG fraction and made specific for human T lymphocytes by means of extensive absorption with all kinds of human blood cells. Lymphocytes were also tested for E-rosette formation with sheep red blood cells (SRBC). In adults, an average of 79% of peripheral blood lymphocytes reacted with the ATC, 14% with the anti-Fab conjugate, 2% with both conjugates and 5% with neither of the conjugates. An average of 76% of peripheral blood lymphocytes (PBL) formed E rosettes. Relative numbers of fluorescent B and T lymphocytes in blood from children showed no significant differences as compared to adults. The percentage of E rosettes in cord blood was lower than in any of the other age groups studied, but only in comparison with the 3–12-month-old age group was the difference significant (P<0·05). In the 3–6-month-old age group, the percentage of fluorescent T lymphocytes was highest and the percentage of fluorescent B lymphocytes lowest, but a significant difference was observed only for the B-lymphocyte percentage compared with the first month of age (P<0·02). Up to 2 years of age, absolute values for total blood lymphocytes, fluorescent T and B lymphocytes and E rosettes were significantly higher (P<0·001) than in the older age groups and adults. After the second year of life, those values were the same as in adults.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisenberg A. C., Bloch K. J., Long J. C., Colvin R. B. Reaction of normal human lymphocytes and chronic lymphocytic leukemia cells with an antithymocyte antiserum. Blood. 1973 Mar;41(3):417–423. [PubMed] [Google Scholar]
- Aiuti F., Lacava V., Garofalo J. A., D'Amelio R., D'Asero C. Surface markers on human lymphocytes: studies of normal subjects and of patients with primary immunodeficiencies. Clin Exp Immunol. 1973 Sep;15(1):43–52. [PMC free article] [PubMed] [Google Scholar]
- Aiuti F., Wigzell H. Function and distribution pattern of human T lymphocytes. I. Production of anti-T lymphocyte specific sera as estimated by cytotoxicity and elimination of function of lymphocytes. Clin Exp Immunol. 1973 Feb;13(2):171–181. [PMC free article] [PubMed] [Google Scholar]
- Bach J. F., Dormont J., Dardenne M., Balner H. In vitro rosette inhibition by antihuman antilymphocyte serum. Correlation with skin graft prolongation in subhuman primates. Transplantation. 1969 Sep;8(3):265–280. doi: 10.1097/00007890-196909000-00008. [DOI] [PubMed] [Google Scholar]
- Bach J. F. Evaluation of T-cells and thymic serum factors in man using the rosette technique. Transplant Rev. 1973;16(0):196–217. doi: 10.1111/j.1600-065x.1973.tb00121.x. [DOI] [PubMed] [Google Scholar]
- Balch C. M., Dagg M. K., Cooper M. D. Cross-reactive T-cell antigens among mammalian species. J Immunol. 1976 Aug;117(2):447–449. [PubMed] [Google Scholar]
- Bobrove A. M., Strober S., Herzenberg L. A., DePamphilis J. D. Identification and quantitation of thymus-derived lymphocytes in human peripheral blood. J Immunol. 1974 Feb;112(2):520–527. [PubMed] [Google Scholar]
- Brier A. M., Chess L., Schlossman S. F. Human antibody-dependent cellular cytotoxicity. Isolation and identification of a subpopulation of peripheral blood lymphocytes which kill antibody-coated autologous target cells. J Clin Invest. 1975 Dec;56(6):1580–1586. doi: 10.1172/JCI108240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G., Greaves M. F. Cell surface markers for human T and B lymphocytes. Eur J Immunol. 1974 Apr;4(4):302–310. doi: 10.1002/eji.1830040414. [DOI] [PubMed] [Google Scholar]
- Brown G., Greaves M. F. Enumeration of absolute numbers of T and B lymphocytes in human blood. Scand J Immunol. 1974;3(2):161–172. doi: 10.1111/j.1365-3083.1974.tb01244.x. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Campbell A. C., Waller C., Wood J., Aynsley-Green A., Yu V. Lymphocyte subpopulations in the blood of newborn infants. Clin Exp Immunol. 1974 Dec;18(4):469–482. [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975 Jun 1;141(6):1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao J. W., Good R. A. Studies of the presence of membrane receptors for complement, IgG and the sheep erythrocyte rosetting capacity on the same human lymphocytes. Eur J Immunol. 1976 Mar;6(3):157–162. doi: 10.1002/eji.1830060304. [DOI] [PubMed] [Google Scholar]
- Chiao J. W., Pantic V. S., Good R. A. Human peripheral lymphocytes bearing both B-cell complement receptors and T-cell characteristics for sheep erythrocytes detected by a mixed rosette method. Clin Exp Immunol. 1974 Dec;18(4):483–490. [PMC free article] [PubMed] [Google Scholar]
- Davis R. H., Galant S. P. Nonimmune rosette formation: a measure of the newborn infant's cellular immune response. J Pediatr. 1975 Sep;87(3):449–452. doi: 10.1016/s0022-3476(75)80658-5. [DOI] [PubMed] [Google Scholar]
- Diaz-Jauanen E., Strickland R. G., Williams R. C. Studies of human lymphocytes in the newborn and the aged. Am J Med. 1975 May;58(5):620–628. doi: 10.1016/0002-9343(75)90497-0. [DOI] [PubMed] [Google Scholar]
- Ferrarini M., Moretta L., Abrile R., Durante M. L. Receptors for IgG molecules on human lymphocytes forming spontaneous rosettes with sheep red cells. Eur J Immunol. 1975 Jan;5(1):70–72. doi: 10.1002/eji.1830050115. [DOI] [PubMed] [Google Scholar]
- Ferrarini M., Tonda G. P., Risso A., Viale G. Lymphocyte membrane receptors in human lymphoid leukemias. Eur J Immunol. 1975 Feb;5(2):89–93. doi: 10.1002/eji.1830050204. [DOI] [PubMed] [Google Scholar]
- Gajl-Peczalska K. J., Park B. H., Biggar W. D., Good R. A. B and T lymphocytes in primary immunodeficiency disease in man. J Clin Invest. 1973 Apr;52(4):919–928. doi: 10.1172/JCI107257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmelig-Meyling F., Van der Ham M., Ballieux R. E. Binding of IgM by human T lymphocytes. Scand J Immunol. 1976;5(5):487–495. doi: 10.1111/j.1365-3083.1976.tb00303.x. [DOI] [PubMed] [Google Scholar]
- Hijmans W., Schuit H. R., Klein F. An immunofluorescence procedure for the detection of intracellular immunoglobulins. Clin Exp Immunol. 1969 Apr;4(4):457–472. [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson V. Technical aspects of the rosette technique for detecting human circulating B and T lymphocytes. Normal values and some remarks on null lymphocytes. Scand J Haematol. 1974;13(5):361–369. [PubMed] [Google Scholar]
- Kersey J. H., Gajl-Peczalska J. T and B lymphocytes in humans. A review. Am J Pathol. 1975 Nov;81(2):446–458. [PMC free article] [PubMed] [Google Scholar]
- Kersey J., Nesbit M., Hallgren H., Sabad A., Yunis E., Gajl-Peczalska K. Evidence for origin of certain childhood acute lymphoblastic leukemias and lymphomas in thymus-derived lymphocytes. Cancer. 1975 Oct;36(4):1348–1352. doi: 10.1002/1097-0142(197510)36:4<1348::aid-cncr2820360424>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Lay W. H., Mendes N. F., Bianco C., Nussenzweig V. Binding of sheep red blood cells to a large population of human lymphocytes. Nature. 1971 Apr 23;230(5295):531–532. doi: 10.1038/230531a0. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J., Cone R. E., Boehmer H. V. Surface immunoglobulins of peripheral thymus-derived lymphocytes. Immunochemistry. 1974 May;11(5):271–277. doi: 10.1016/0019-2791(74)90206-7. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. Lymphocyte surface immunoglobulins. Science. 1975 Oct 3;190(4209):20–29. doi: 10.1126/science.1101378. [DOI] [PubMed] [Google Scholar]
- Moretta L., Ferrarini M., Durante M. L., Mingari M. C. Expression of a receptor for IgM by human T cells in vitro. Eur J Immunol. 1975 Aug;5(8):565–569. doi: 10.1002/eji.1830050812. [DOI] [PubMed] [Google Scholar]
- Moroz C., Hahn Y. Cell-surface immunoglobulin human thymus cells and its biosynthesis in vitro. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3716–3720. doi: 10.1073/pnas.70.12.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen F. L., Fanger M. W. Studies on the human T-lymphocyte population - IV. The isolation of T-lymphocyte antigens from peripheral lymphocytes. Immunochemistry. 1976 Feb;13(2):121–127. doi: 10.1016/0019-2791(76)90279-2. [DOI] [PubMed] [Google Scholar]
- Owen J. J., Raff M. C. Studies on the differentiation of thymus-derived lymphocytes. J Exp Med. 1970 Dec 1;132(6):1216–1232. doi: 10.1084/jem.132.6.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmann P., Perlmann H., Wigzell H. Lymphocyte mediated cytotoxicity in vitro. Induction and inhibition by humoral antibody and nature of effector cells. Transplant Rev. 1972;13:91–114. doi: 10.1111/j.1600-065x.1972.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Roelants G. E., Loor F., von Boehmer H., Sprent J., Hägg L. B., Mayor K. S., Rydén A. Five types of lymphocytes (Ig-theta-, Ig-theta+weak, Ig-theta+strong, Ig+theta- and Ig+theta+) characterized by double immunofluorescence and electrophoretic mobility. Organ distribution in normal and nude mice. Eur J Immunol. 1975 Feb;5(2):127–131. doi: 10.1002/eji.1830050211. [DOI] [PubMed] [Google Scholar]
- Roelants G. E., Mayor K. S., Hägg L. B., Loor F. Immature T lineage lymphocytes in athymic mice. Presence of TL, lifespan and homeostatic regulation. Eur J Immunol. 1976 Feb;6(2):75–81. doi: 10.1002/eji.1830060202. [DOI] [PubMed] [Google Scholar]
- Rádl J., Skvaril F., Masopust J., Kithier K. Quantitative estimation of human immunoglobulins. II. The development of serum immunoglobulin levels in man. J Hyg Epidemiol Microbiol Immunol. 1970;14(4):488–498. [PubMed] [Google Scholar]
- Rádl J., van den Berg P., Voormolen M., Hendriks W. D., Schaefer U. W. Homogeneous immunoglobulins in sera of rhesus monkeys after lethal irradiation and bone marrow transplantation. Clin Exp Immunol. 1974 Feb;16(2):259–266. [PMC free article] [PubMed] [Google Scholar]
- Smith M. A., Evans J., Steel C. M. Age-related variation in proportion of circulating T cells. Lancet. 1974 Oct 19;2(7886):922–924. doi: 10.1016/s0140-6736(74)91130-1. [DOI] [PubMed] [Google Scholar]
- Smith R. W., Terry W. D., Buell D. N., Sell K. W. An antigenic marker for human thymic lymphocytes. J Immunol. 1973 Mar;110(3):884–887. [PubMed] [Google Scholar]
- Touraine J. L., Touraine F., Kiszkiss D. F., Choi Y. S., Good R. A. Heterologous specific antiserum for identification of human T lymphocytes. Clin Exp Immunol. 1974 Apr;16(4):503–520. [PMC free article] [PubMed] [Google Scholar]
- Vossen J. M. Membrane-associated immunoglobulin determinants on bone marrow and blood lymphocytes in the pediatric age group and on fetal tissues. Ann N Y Acad Sci. 1975 Jun 30;254:262–279. doi: 10.1111/j.1749-6632.1975.tb29176.x. [DOI] [PubMed] [Google Scholar]
- Whiteside T. L., Rabin B. S. Surface immunoglobulin on activated human peripheral blood thymus-derived cells. J Clin Invest. 1976 Mar;57(3):762–771. doi: 10.1172/JCI108335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worth H. H., Cooper A. G., Brown M. C. Inhibition of human lymphocyte rosetting by anti-T sera. Nat New Biol. 1973 May 23;243(125):109–111. [PubMed] [Google Scholar]
- Wybran J., Carr M. C., Fudenberg H. H. The human rosette-forming cell as a marker of a population of thymus-derived cells. J Clin Invest. 1972 Oct;51(10):2537–2543. doi: 10.1172/JCI107069. [DOI] [PMC free article] [PubMed] [Google Scholar]


