Abstract
Highly purified Fc receptor-negative T lymphocytes were obtained by filtration of human blood mononuclear cells through Ig–anti-Ig columns. Monocyte-enriched cells were isolated by density centrifugation in bovine serum albumin solutions. The proliferative in vitro response of the purified T lymphocytes was investigated with and without the addition of monocyte-enriched cells, after stimulation by allogeneic cells, phytohaemagglutinin (PHA), pokeweed mitogen (PWM), purified protein derivative (PPD), Candida albicans, Staphylococcus aureus, E. coli and tetanus toxoid.
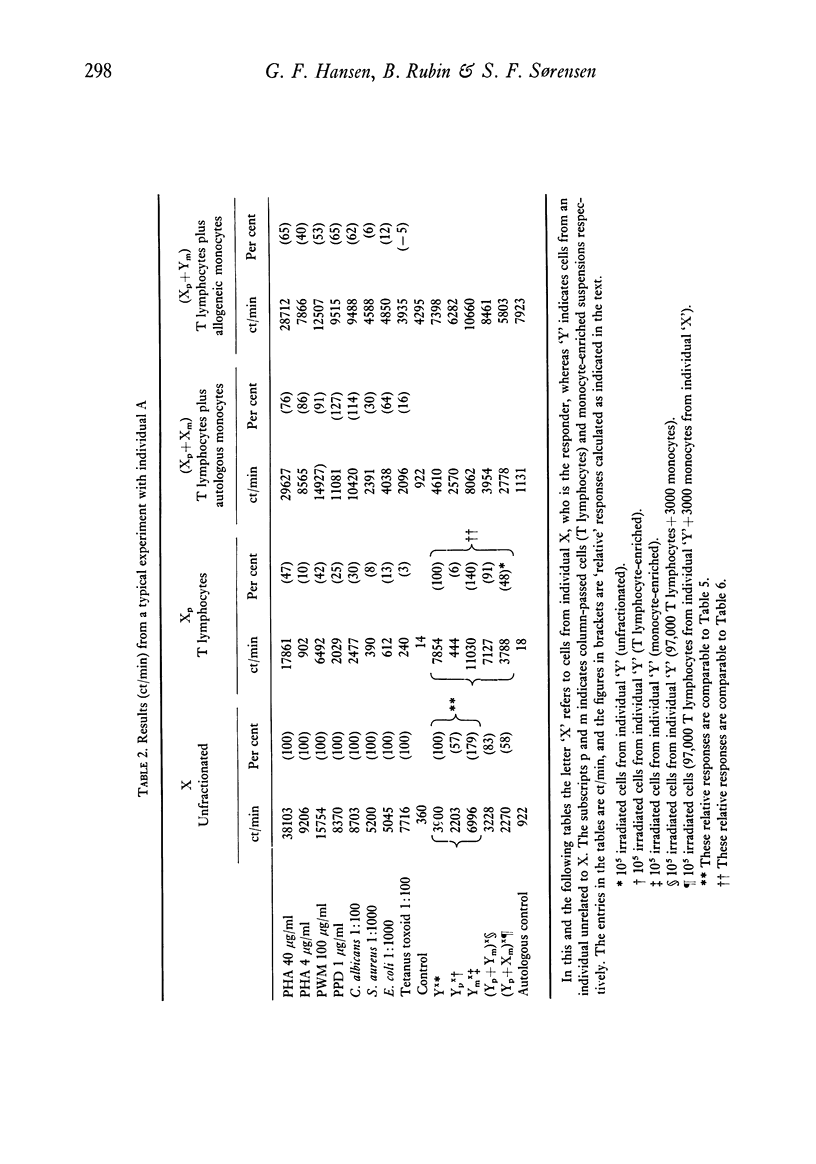
The results were as follows:
(1) Fc receptor-negative T lymphocytes respond autonomously, and are the main source of proliferative cells found after stimulation by allogeneic cells and optimal doses of PHA, PWM and PPD. Addition of monocyte-enriched cells increases the responses, presumably by a non-specific feeder-effect in the cultures.
(2) Stimulation of Fc receptor-negative T lymphocytes by C. albicans, S. aureus, E. coli and tetanus toxoid requires the presence of autologous monocytes in the cultures, whereas allogeneic monocytes do not support the responses.
(3) Fc receptor-negative T lymphocytes are the main proliferating cells found after stimulation by C. albicans, whereas other cells (B-lymphocytes and/or Fc receptor-positive T lymphocytes) may be responsible for a substantial part of the proliferation elicited by S. aureus, E. coli and tetanus toxoid.
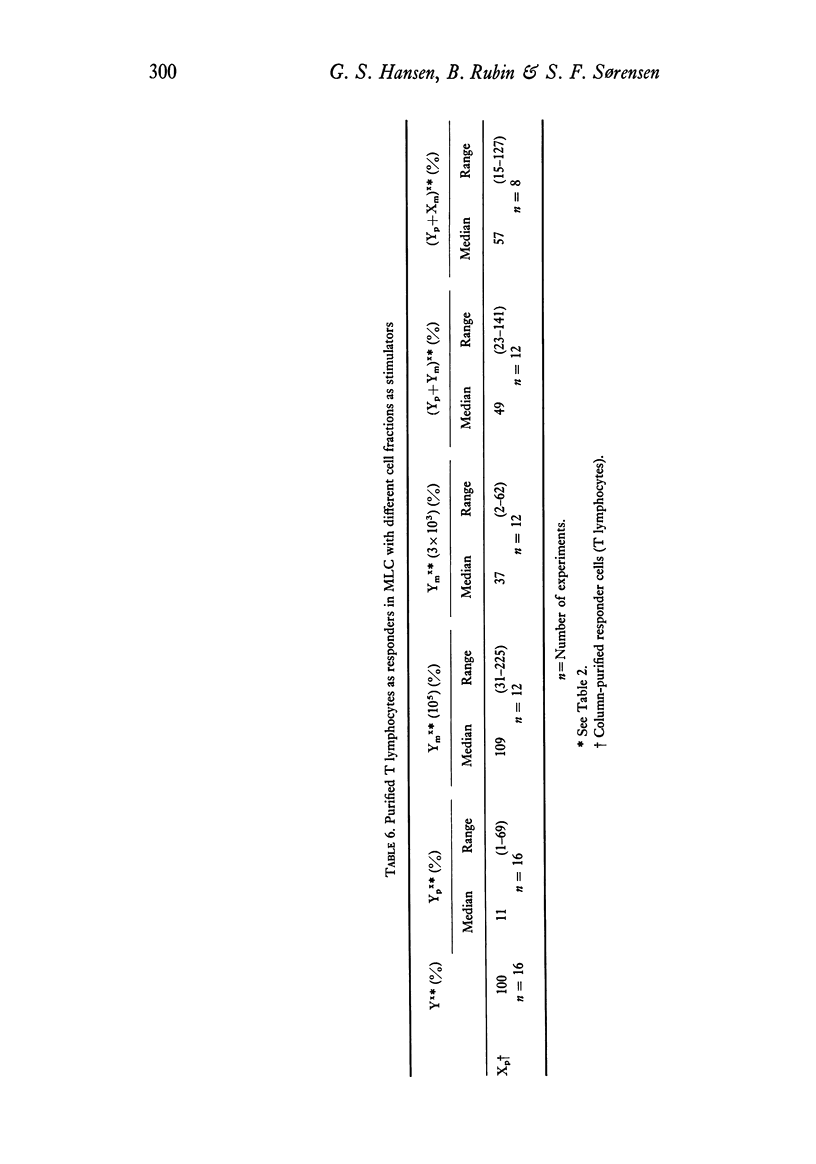
(4) Fc receptor-negative T lymphocytes can stimulate allogeneic cells in mixed lymphocyte culture, but monocyte-enriched cells and unfractionated mononuclear cells are better in this respect.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach F. H., Alter B. J., Solliday S., Zoschke D. C., Janis M. Lymphocyte reactivity in vitro. II. Soluble reconstituting factor permitting response of purified lymphocyte. Cell Immunol. 1970 Jul;1(2):219–227. doi: 10.1016/0008-8749(70)90009-2. [DOI] [PubMed] [Google Scholar]
- Berman M., Puryear K., Argyris B. F. In vitro recognition of alloantigens: nature of responding and stimulating cells. Cell Immunol. 1976 Apr;23(1):126–139. doi: 10.1016/0008-8749(76)90177-5. [DOI] [PubMed] [Google Scholar]
- Blomgren H. Role of B cells in the expression of the PPD response of human lymphocytes in vitro. Scand J Immunol. 1975 Sep;4(5-6):499–510. doi: 10.1111/j.1365-3083.1975.tb02655.x. [DOI] [PubMed] [Google Scholar]
- Chess L., MacDermott R. P., Schlossman S. F. Immunologic functions of isolated human lymphocyte subpopulations. II. Antigen triggering of T and B cells in vitro. J Immunol. 1974 Oct;113(4):1122–1127. [PubMed] [Google Scholar]
- Fudenberg H., Good R. A., Goodman H. C., Hitzig W., Kunkel H. G., Roitt I. M., Rosen F. S., Rowe D. S., Seligmann M., Soothill J. R. Primary immunodeficiencies. Report of a World Health Organization Committee. Pediatrics. 1971 May;47(5):927–946. [PubMed] [Google Scholar]
- Hertel-Wulff B., Rubin B. Lymphocyte proliferation in vitro induced by soluble protein antigens. II. Cellular requirements. Eur J Immunol. 1976 Jun;6(6):418–424. doi: 10.1002/eji.1830060608. [DOI] [PubMed] [Google Scholar]
- Huber H., Fudenberg H. H. Receptor sites of human monocytes for IgG. Int Arch Allergy Appl Immunol. 1968;34(1):18–31. doi: 10.1159/000230091. [DOI] [PubMed] [Google Scholar]
- Jensen B., Kurpisz M., Rubin B. Antigen specific lymphocyte activity in vitro by peripheral blood leucocytes from Mantoux positive and negative human beings. I. Comparison of quantitative and qualitative differences in the PPD-specific lymphoproliferative response of lymphocytes from the two kinds of donors. Clin Exp Immunol. 1977 Feb;27(2):303–312. [PMC free article] [PubMed] [Google Scholar]
- Lipsky P. E., Ellner J. J., Rosenthal A. L. Phytohemagglutinin-induced proliferation of guinea pig thymus-derived lymphocytes. I. Accessory cell dependence. J Immunol. 1976 Mar;116(3):868–875. [PubMed] [Google Scholar]
- Lohrmann H. P., Novikovs L., Graw R. G., Jr Cellular interactions in the proliferative response of human T and B lymphocytes to phytomitogens and allogeneic lymphocytes. J Exp Med. 1974 Jun 1;139(6):1553–1567. doi: 10.1084/jem.139.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohrmann H. P., Novikovs L., Graw R. G., Jr Stimulatory capacity of human T and B lymphocytes in the mixed leukocyte culture. Nature. 1974 Jul 12;250(462):144–146. doi: 10.1038/250144a0. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J., Leventhal B. G., Hersh E. M. The transformation of column-purified lymphocytes with nonspecific and specific antigenic stimuli. J Immunol. 1968 Aug;101(2):262–267. [PubMed] [Google Scholar]
- Phillips B., Roitt I. M. Evidence for transformation of human B lymphocytes by PHA. Nat New Biol. 1973 Feb 21;241(112):254–256. doi: 10.1038/newbio241254a0. [DOI] [PubMed] [Google Scholar]
- Rosenthal A. S., Shevach E. M. Function of macrophages in antigen recognition by guinea pig T lymphocytes. I. Requirement for histocompatible macrophages and lymphocytes. J Exp Med. 1973 Nov 1;138(5):1194–1212. doi: 10.1084/jem.138.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin B., Wigzell H. Lymphocyte proliferation in vitro induced by hapten autologous protein conjugates. I. A study on the class of lymphocytes responding in vitro and on the nature and specificity of their receptors. J Exp Med. 1974 Mar 1;139(3):732–753. doi: 10.1084/jem.139.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtke J. R., Hatfield S. Activation of purified human thymus-derived (T) cells by mitogens. II. Monocyte- macrophage potentiation of mitogen-induced DNA synthesis. J Immunol. 1976 Feb;116(2):357–362. [PubMed] [Google Scholar]
- Zeylemaker W. P., Roos M. T., Meyer C. J., Schellekens P. T., Eijsvoogel V. P. Separation of human lymphocyte subpopulations. I. Transformation characteristics of rosette-forming cells. Cell Immunol. 1974 Dec;14(3):346–358. doi: 10.1016/0008-8749(74)90184-1. [DOI] [PubMed] [Google Scholar]


