Abstract
Introduction
Lung collapse is a contributory factor in the hypoxaemia that is observed after open endotracheal suctioning (ETS) in patients with acute lung injury and acute respiratory distress syndrome. Lung recruitment (LR) manoeuvres may be effective in rapidly regaining lung volume and improving oxygenation after ETS.
Materials and method
A prospective, randomized, controlled study was conducted in a 15-bed general intensive care unit at a university hospital. Eight consecutive mechanically ventilated patients with acute lung injury or acute respiratory distress syndrome were included. One of two suctioning procedures was performed in each patient. In the first procedure, ETS was performed followed by LR manoeuvre and reconnection to the ventilator with positive end-expiratory pressure set at 1 cmH2O above the lower inflexion point, and after 60 min another ETS (but without LR manoeuvre) was performed followed by reconnection to the ventilator with similar positive end-expiratory pressure; the second procedure was the same as the first but conducted in reverse order. Before (baseline) and over 25 min following each ETS procedure, partial arterial oxygen tension (PaO2) and end-expiratory lung volume were measured.
Results
After ETS, PaO2 decreased by 4.3(0.9–9.7)kPa (median and range; P < 0.005). After LR manoeuvre, PaO2 recovered to baseline. Without LR manoeuvre, PaO2 was reduced (P = 0.05) until 7 min after ETS. With LR manoeuvre end-expiratory lung volume was unchanged after ETS, whereas without LR manoeuvre end-expiratory lung volume was still reduced (approximately 10%) at 5 and 15 min after ETS (P = 0.01).
Discussion
A LR manoeuvre immediately following ETS was, as an adjunct to positive end-expiratory pressure, effective in rapidly counteracting the deterioration in PaO2 and lung volume caused by open ETS in ventilator-treated patients with acute lung injury or acute respiratory distress syndrome.
Keywords: acute respiratory distress syndrome, alveolar recruitment, atelectasis, hypoxaemia, suction/instrumentation
Introduction
After discontinuation of positive end-expiratory pressure (PEEP), lung collapse occurs rapidly in ventilator-treated patients with acute respiratory distress syndrome (ARDS) [1]. Endotracheal suctioning (ETS), which is a common procedure in patients with acute lung injury (ALI) and ARDS, abolishes the positive airway pressure and even may generate negative pressure, promoting de-recruitment and hypoxaemia [2].
The most common method used to mitigate the reduction in oxygenation induced by suctioning is to increase the fractional inspired oxygen (FiO2) [3,4,5]. This strategy is often effective in patients with less severe lung diseases, but is less efficacious in patients with ARDS with high shunt fractions [6]. In addition, a high FiO2 may augment lung collapse by causing absorption atelectasis [7,8,9]. It has recently been suggested that a closed suction system may be effective in preventing suctioning-induced decreases in lung volume and oxygenation. In fact, Pesenti and coworkers [10] found no reduction in end-expiratory lung volume (EELV) or arterial oxygen saturation in patients with ALI and ARDS after suctioning with such a system. Significant drawbacks with closed suction systems include risk for producing high negative pressures and reduced efficacy in removing thick secretions from the airways [2,11,12]. Brochard and coworkers [13] showed that lung volume and arterial oxygenation could be maintained during open suctioning by using constant flow insufflation. This method appears to be effective but necessitates use of a special endotracheal tube. Another measure to counteract suctioning-induced hypoxaemia is hyperinflation of the lungs. This is usually performed by administering large breaths using an anaesthetic balloon, without attention to monitoring of levels, duration or maintenance of the end-inspiratory and end-expiratory pressures [14,15].
Because de-recruitment occurs during and after suctioning, a plausible method to mitigate hypoxaemia is to re-recruit collapsed lung using a lung recruitment (LR) manoeuvre. Indeed, using computed tomography, Lu and coworkers [16] showed that LR manoeuvres are effective in resolving atelectasis and improving oxygenation after ETS in sheep with normal lungs. However, as far as we know, this has not been verified in patients with ALI and ARDS. The present study was therefore conducted to examine the additive effect of LR manoeuvre to adequate PEEP on lung volumes and oxygenation after a standardized open ETS procedure in eight mechanically ventilated patients with ALI or ARDS.
Materials and method
Patients
The present study was approved by the local Human Ethics Committee and informed consent was obtained from next of kin. After a power analysis (see Statistical analysis, below), eight patients with ALI or ARDS requiring mechanical ventilation were enrolled [17]. Exclusion criteria were pneumothorax, documented history of chronic obstructive lung disease, haemodynamic instability and a contraindication to deep sedation. The patients (Table 1) were studied in the supine position, with the upper part of the body slightly higher than the lower, and ventilated via an endotracheal tube (size 7.5–8; Mallincrodt, Hazelwood, MO, USA) either in the volume-controlled or pressure-controlled mode (Servo Ventilator 900C; Siemens-Elema, Solna, Sweden; Table 2). Sedation was performed with continuous intravenous infusion of propofol (50–150 mg/h) and intermittent intravenous morphine (1–5 mg). The infusion rate of propofol was adjusted so that the patient exhibited no spontaneous breathing efforts during the study. If signs of arousal appeared, then an intravenous bolus of 20–50 mg propofol was administered. Approximately 5 min before start of the study the patient was given an intravenous bolus of 30–50 mg propofol. Muscle relaxants were not used. If the physician in charge considered them necessary, fluids and blood products were administered. The patients were monitored by electrocardiography, continuous invasive blood pressure monitoring and pulse oximetry (HP model 68S, Viridia CMS; Hewlett-Packard, Boeblingen, Germany). We considered a transient decrease in arterial saturation estimated by pulse oximetry (SpO2) to 80% to be acceptable, without constituting a breach of protocol.
Table 1.
Patient characteristics at inclusion and patient outcome
| Patient number | Age (years) | Sex (F/M) | Weight (kg) | EELV (ml) | PEEP (cmH2O) | PaO2/ FiO2 (kPa) | LIS | Underlying disease | Cause of acute lung injury | MV (days) | Outcome |
| 1 | 75 | M | 69 | 2284 | 10 | 36.8 | 2.3 | Secondary lung cancer | Pneumonia | 2 | S |
| 2 | 69 | F | 75 | 1272 | 11 | 20.0 | 2.5 | Liver cirrhosis, colectomy | Sepsis, pneumonia | 7 | D |
| 3 | 76 | M | 96 | 1513 | 8 | 9.0 | 2.7 | AAA, secondary bowel ischaemia | Sepsis | 2 | S |
| 4 | 67 | M | 85 | 1309 | 9 | 19.4 | 3.3 | Fasciitis | Aspiration pneumonia | 5 | D |
| 5 | 66 | M | 95 | 2245 | 10 | 19.6 | 2.7 | - | Pneumonia | 2 | S |
| 6 | 81 | M | 109 | 1639 | 13 | 23.3 | 2.5 | AAA | SIRS | 3 | S |
| 7 | 65 | F | 72 | 949 | 11 | 17.5 | 3.0 | TAA | Sepsis | 3 | S |
| 8 | 58 | F | 63 | 1233 | 15 | 15.4 | 3.3 | CABG | Pneumonia | 5 | S |
| Mean ± SD | 70 ± 7 | 3/5 | 83 ± 16 | 1550 ± 480 | 11 ± 2 | 20.1 ± 8 | 2.8 ± 0.4 | - | - | 3.6 ± 1.8 | - |
The two patients who died did so 22 and 31 days after the study in multiple organ dysfunction syndrome after discontinuation of active life support. AAA, abdominal aortic aneurysm; CABG, coronary artery bypass graft surgery; D, died; EELV, end-expiratory lung volume; LIS, lung injury score; MV, days of mechanical ventilation before measurements; S, survived; SIRS, systemic inflammatory response syndrome; TAA, thoracic aortic aneurysm.
Table 2.
Ventilatory parameters at baseline of the two different suction procedures
| Parameter | ETS+LR | ETS-LR |
| PaO2/FiO2 (kPa) | 20 (11–36) | 23 (12–48) |
| Set PEEP (cmH2O) | 12 (9–16) | 12 (9–16)) |
| Intrinsic PEEP (cmH2O) | 1 (0–3) | 1 (0–3) |
| Vt/kg (ml/kg) | 6 (5–9) | 6 (5–9) |
Note that the baseline values are after the standardization procedure (see text). ETS+LR, endotracheal suctioning followed by a lung recruitment manoeuvre; ETS-LR, endotracheal suctioning without a following lung recruitment manoeuvre; FiO2, fractional inspired oxygen; set PEEP; set positive end-expiratory pressure; Intrinsic PEEP, PEEP above set PEEP after an expiratory hold; Vt, tidal volume.
Protocol
A crossover design was employed. Before the start of the study, the patients were randomized to one of two sequences of two open ETS procedures. In the first sequence patients were first subjected to ETS followed by an immediate LR manoeuvre (ETS+LR), and then after 60 min they were subjected to another ETS procedure but without a LR manoeuvre (ETS-LR). In the second sequence patients were first subjected to ETS without a LR manoeuvre, and then after 60 min they were subjected to another ETS procedure but immediately followed by a LR manoeuvre (i.e. the same as the first sequence but in reverse order). Following each ETS procedure, measurements were over a period of 25 min.
After a 30 min standardization period (see below), ETS+LR consisted of disconnection of the tube from the ventilator, then ETS, followed by reconnection to the ventilator with the set PEEP and an immediate LR manoeuvre. After a 30 min standardization period (see below), ETS-LR consisted of disconnection of the endotracheal tube from the ventilator, then ETS, followed by reconnection to the ventilator with the set PEEP (Fig. 1).
Figure 1.
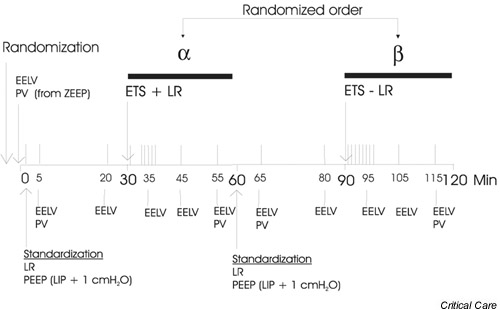
Timeline of the study in minutes. The order of the two suctioning procedures α and β was randomized. The vertical lines above the timeline indicate blood gas samplings. ETS+LR, endotracheal suctioning followed by a lung recruitment manoeuvre; ETS-LR, endotracheal suctioning without a following lung recruitment manoeuvre; EELV, end-expiratory lung volume; LIP, lower inflection point; LR, lung recruitment manoeuvre; PEEP, positive end-expiratory pressure; PV, inspiratory pressure–volume curve.
ETS was performed by inserting the tip of a suction catheter (size 14, Oppo-cath I; Pennine Healthcare, Derby, UK) 2 cm below the distal end of the endotracheal tube. In order to mimic the suctioning routines in our intensive care unit, the suctioning pressure at the wall inlet was set to generate a peak pressure of 400 mmHg when the catheter was totally occluded. The trachea was suctioned three times for 5 s with an interval of 10 s between each suctioning, during which the catheter was changed. This resulted in a 35 s period of disconnection from the ventilator.
The LR manoeuvre consisted of two hyperinflations using the continuous positive airways pressure function of the ventilator to an airway pressure of 45 cmH2O for 20 s, with an interval of 1 min in between [18].
After randomization the EELV was measured, an airway pressure–lung volume curve was obtained (from zero end-expiratory pressure in order to identify the lower inflexion point [LIP]) and blood gases were sampled and analyzed using a blood gas analyzer (ABL 725; Radiometer, Copenhagen, Denmark). FiO2 was adjusted if partial arterial oxygen tension (PaO2) was below 10 kPa, and was then kept unchanged during the study. EELV was measured by a wash-in/washout method using sulphur hexafluoride (SF6) as the tracer gas [19]. This measurement technique can be used without disconnecting the patient from the ventilator, with pressure-controlled or volume-controlled ventilation, and with FiO2 up to 0.995. The measurement system consists of a ventilator (Servo Ventilator 900C; Siemens-Elema), a tracer gas dispensing valve, an in-line infrared SF6 transducer/analyser, and a computer that governs the dispensing valve and uses the SF6 signal from the transducer/analyzer and the flow signals from the ventilator for calculations of lung volume. The tracer gas is insufflated via the dispensing valve in proportion to the inspiratory flow, so that a uniform concentration of 0.5% is achieved. When the alveolar concentration is stable, as assessed by a constant expiratory plateau concentration from breath to breath, wash-in is stopped and washout is started. Washout is considered complete when the mean end-tidal concentration is less than 0.005% in the last five breaths. The SF6 flow in the expired breath is integrated and the accumulated amount of SF6 during washout is calculated (ΣSF6) and EELV is obtained form the following equation: EELV = ΣSF6/%SF6 at the end of wash-in.
The inspiratory pressure–volume curves were obtained using the computerized method described by Jonson and coworkers [20,21]. After a prolonged (6 s) expiratory pause, an inspiratory pressure–volume curve is recorded during slow insufflation. The flow is integrated to obtain the volume, after correcting for the compliance of the ventilator tubing. The pressure drop caused by the resistance of the tracheal tube (which is measured in vitro) is subtracted from the pressure measured in the ventilator. The pressure–volume curve is then mathematically described according to the principle of Newton–Raphson to a three-segment model: a lower nonlinear segment, over which compliance increases linearly with volume; a middle linear segment, with a constant compliance; and an upper nonlinear segment, over which compliance falls linearly with volume. The transition between the lower and middle segment is defined as the LIP. Before measurement of EELV and the pressure–volume curves in each patient, the measurement equipment was calibrated. The volumes presented are converted from ambient temperature pressure saturated (ATPS) to body temperature pressure saturated (BTPS).
With regard to the standardization period, before each ETS, lung volume was standardized via a LR manoeuvre, after which the patient was ventilated with PEEP set at 1 cmH2O above LIP for 30 min. This PEEP level was used during the whole study. Also, the end-inspiratory plateau pressures, tidal volumes and rates were kept constant during the study (Table 2). During the standardization period blood gases were sampled and EELV was measured at 5 and 20 min (baseline values) after the LR manoeuvre. In addition, a pressure–volume curve (with the starting pressure at PEEP, in order to prevent de-recruitment during the procedure) was obtained at 5 min after the LR manoeuvre (Fig. 1).
After each ETS, measurements of EELV were taken at 5, 15 and 25 min, and blood gases were sampled at 0, 1, 2, 3, 4, 5, 6, 7, 15 and 25 min. However, because of logistical factors, after ETS+LR blood gases were not sampled during the LR manoeuvre (i.e. at 1 and 2 min). A pressure–volume curve was obtained (from the PEEP) at 25 min after ETS. The blood gas samples taken during the first 7 min after ETS were stored on ice and analyzed after approximately 10 min. The blood gas samples taken at 15 and 25 min were analyzed immediately.
Statistical analysis
A power analysis (assuming that the difference in change in PaO2 at 5 min would be 30 ± 15%) indicated that eight patients needed to be included (with α = 0.05 and 1 - β = 0.80, using a crossover design). Because we were interested in changes in oxygenation and lung volume during the procedure, the values obtained were normalized to the value just before each ETS procedure (baseline values). Changes within the procedures were assessed using analysis of variance and a post hoc analysis (PLSD), and changes between the procedures at similar points in time were assessed using the Wilcoxon signed rank test. P < 0.05 was considered statistically significant. The differences in volume at similar pressures on the pressure–volume curves were compared using the Kruskall–Wallis test. Data are presented as mean ± SD, if not otherwise indicated.
Results
The demographic data for the patients, and their underlying conditions and initial respiratory parameters are presented in Table 1. Seven patients had ARDS and one had ALI at inclusion [17]. Lung injury score [22] was 2.7 (2.3–3.3) (median [range]).
Haemodynamics
During LR manoeuvres, the decrease in blood pressure never exceeded 14 mmHg and mean arterial pressure was always greater than 50 mmHg (Table 3). No arrhythmias were observed. Also, when ETS was performed, no arrhythmias and no major changes in pulse rate or blood pressure occurred in any patient (Table 3).
Table 3.
Haemodynamic parameters at baseline and 2 min after the two different suction procedures
| ETS+LR | ETS-LR | |||
| Parameter | Baseline | 2 min after ETS + LR | Baseline | 2 min after ETS-LR |
| HR (beats/min) | 90 ± 8 | 96 ± 9* | 90 ± 7 | 91 ± 7 |
| CVP (mmHg) | 15 ± 4 | 18 ± 5 | 15 ± 4 | 15 ± 4 |
| MAP (mmHg) | 76 ± 8 | 68 ± 8 | 76 ± 9 | 72 ± 10 |
Values are expressed as mean ± SD. *P < 0.05 between the two procedures. CVP, central venous pressure; ETS+LR, endotracheal suctioning followed by a lung recruitment manoeuvre; ETS-LR, endotracheal suctioning without a following lung recruitment manoeuvre; HR: heart rate; MAP, mean systemic arterial pressure.
Oxygenation
In all but one patient, SpO2 was above 80% throughout the period of study. The patient (no. 3) was randomly assigned to start with ETS+LR. Arterial oxygen tension and FiO2 were 11 kPa and 1.0, respectively. Immediately after the second intervention (i.e. ETS-LR), a SpO2 of 75% was observed. The saturation increased within 1 min to above 80% without intervention, and during the subsequent 4 min to 90%. PaO2 obtained immediately after ETS was 5.1 kPa. Because the blood gases were not analyzed until 8–10 mins after ETS, we were not aware of this low value. At the time when the blood gases were analysed, PaO2 had recovered well (PaO2 obtained at 7 min was 8.9 kPa). However, PaO2 was 6.4, 6.7, 6.7 and 7.0 kPa at 1, 2, 3 and 4 min after ETS.
At inclusion PaO2 was 11.4 ± 3.1 kPa, and immediately before suctioning (baseline PaO2) it was about 2 kPa higher. The changes, presented as percentage of baseline PaO2, during the study are shown in Fig. 2. Immediately after suctioning, PaO2 decreased by 31.7 ± 13.3% (P < 0.05) and 43.0 ± 9.4% (P = 0.0001) of baseline with ETS+LR and ETS-LR, respectively (difference between the two interventions not significant). This corresponds to a median (range) decrease in PaO2 by 4.3(0.9–9.7)kPa (P < 0.005) with both procedures. The lowest PaO2 was 5.1 kPa (see above). With the LR manoeuvre (ETS+LR), PaO2 returned to baseline (100.3 ± 40%) at the first blood gas sample taken after LR manoeuvre (at 3 min) and increased to 121.8 ± 23% at 7 min. Without a LR manoeuvre (ETS-LR), PaO2 did not return to baseline until 7 min after ETS. At 7, 15 and 25 min PaO2 was 88 ± 13%, 91.5 ± 14.3% and 97.2 ± 16.8% of baseline, respectively. After the LR manoeuvre there was a significant difference between the two procedures until 7 min after ETS.
Figure 2.
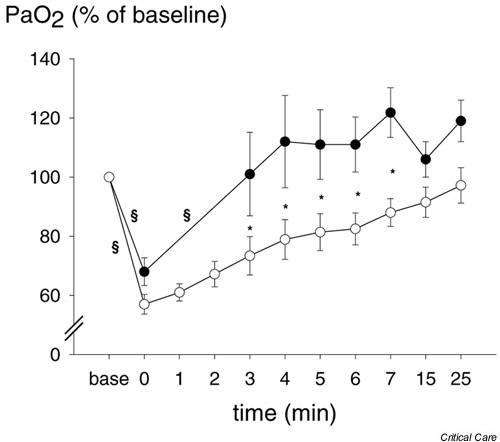
Arterial partial oxygen tension (PaO2), expressed as percentage of baseline, at time points before (baseline) and after the two endotracheal suctioning procedures. Endotracheal suctioning (●) with and (○) without a following lung recruitment manoeuvre. Values are expressed as means ± SEM (bars). *P < 0.05 between the two procedures, §P < 0.05, within the procedures between the measurements.
End-expiratory lung volume
EELV was 1550 ± 480 ml on 8–15 cmH2O PEEP (Table 1) at inclusion, and increased after lung volume standardization (see above) to 1677 ± 618 ml and 1719 ± 571 ml for ETS+LR and ETS-LR, respectively (difference between the two procedures was not significant). Because of the washin/washout SF6 technique, EELV could not be measured until 5 min after the ETS procedure. With the LR manoeuvre (ETS+LR) EELV was similar to baseline at all measurement time points (5, 15 and 25 min after ETS; Fig. 3). Without a LR manoeuvre (ETS-LR) EELV was reduced by 11 ± 10% at 5 min (P < 0.001) and 9 ± 6% at 15 min (P < 0.02). After suctioning, EELV was significantly different between ETS+LR and ETS-LR at all measurement points.
Figure 3.
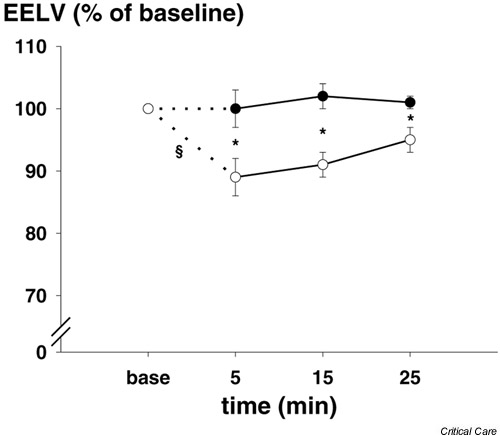
End-expiratory lung volume (EELV) expressed as percentage of baseline, at time points before (baseline) and after the two endotracheal suctioning procedures. Endotracheal suctioning (●) with and (○) without a following lung recruitment manoeuvre. Values are expressed as means ± SEM (bars). *P < 0.05 between the two procedures, §P < 0.01, within the procedures between the measurements.
Mechanics of the respiratory system
At inclusion, the maximal slope of the pressure–volume curves (maximal compliance of the respiratory system) was 32 ml/cmH2O. LIP could be identified in all patients and was located at (median [range]) 11(8–15)cmH2O airway pressure. The pressure–volume curves shown in Fig. 4 were normalized to absolute lung volume at 17.5 cmH2O of airway pressure, in order to integrate all patients, including those with high PEEP, in the calculation of the curves. The pressure–volume curves for ETS+LR and ETS-LR obtained during the standardization period (baseline curves) were almost identical. The pressure–volume curve obtained at 25 min after ETS with the LR manoeuvre (ETS+LR) was similar to the baseline curve but had a tendency to shift upward at high pressures. The maximal slope was 33.3 ± 14.6 ml/cmH2O and 35.9 ± 14.7 ml/cmH2O for the baseline and the 25 min curve, respectively (not significant). The pressure–volume curve obtained at 25 min after ETS without a LR manoeuvre (ETS-LR) was located at lower lung volumes (P < 0.05), and tended to shift downward as compared with the baseline curve. The maximal slope was 35.7 ± 14.6 ml/cmH2O and 32.3 ± 14.7 ml/cmH2O (P < 0.05) for the baseline and 25 min curves, respectively.
Figure 4.
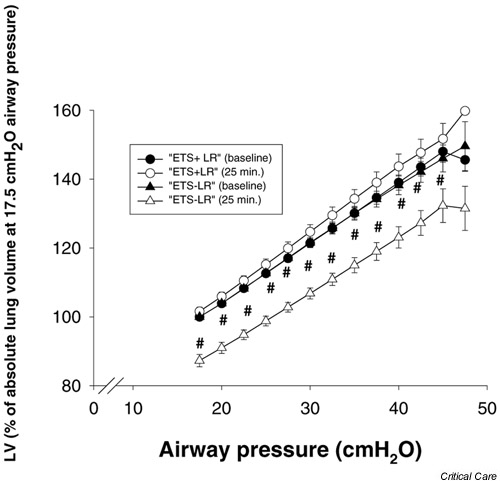
Pressure volume–curves at baseline and at 25 min after the two procedures of endotracheal suctioning. The volumes are normalized to the absolute lung volume at an airway pressure of 17.5 cmH2O at baseline. Values are expressed as means ± SEM (bars). #P < 0.05 between ETS-LR baseline curve and ETS-LR 25 min curve. ETS+LR, endotracheal suctioning followed by a lung recruitment manoeuvre; ETS-LR, endotracheal suctioning without a following lung recruitment manoeuvre.
Discussion
In the present study we demonstrated that a LR manoeuvre, as an additive measure to PEEP after open ETS in mechanically ventilated patients with ALI and ARDS, was well tolerated and produced a rapid recovery in EELV, compliance of the respiratory system and PaO2. In addition, we confirmed that open ETS per se may result in a significant drop in oxygenation and in lung volume.
We studied the effect of the open suctioning procedure used clinically in our unit. The suction pressure was -400 mmHg, which is more than is recommended in some guidelines, but is not uncommon clinical practice in Scandinavia. In the study we checked that the endotracheal tube was not occluded by secretions around the suction catheter, and therefore a marked pressure drop could not occur in the airways. In a lung model test, similar suction pressure gave negative pressures of -17 and -14 cmH2O at the 'tracheal level' for endotracheal tubes with internal diameters of 7.5 and 8 mm, respectively (Fig. 5). Although we cannot exclude the possibility that the level of suction pressure might have contributed, we believe that disconnection from positive airway pressure was the major reason for the decrease in lung volume and PaO2 found in the study. This notion is in accordance with the study conducted by Pesenti and coworkers [10], who found a marked immediate decrease in lung volume (as measured using respiratory inductive plethysmography) when the endotracheal tube was disconnected, followed by a less pronounced decrease at the start of suctioning [10]. In fact, in an experimental ARDS model using computed tomography, Neumann and coworkers [1] showed that major lung collapse occurred within 0.6 s after opening the endotracheal tube to the atmosphere. Moreover, in other studies using lesser suction pressures and duration of suctioning procedure [13,23,24], the reductions in PaO2 and saturation are similar to those reported here.
Figure 5.
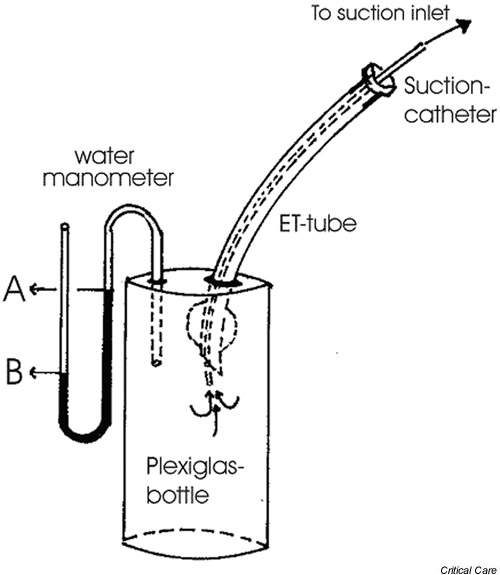
Setup for the lung model for measurement of suction pressure. The test device consisted of a Plexiglas bottle with a connected water manometer. The endotracheal (ET) tube was inserted in the bottle through an opening at the top and the opening was sealed airtight thereafter. The tip of the suctioning catheter was introduced through the ET tube to 2 cm below the distal end of the ET tube. The catheter was then connected to a suction pressure of -400 mmHg at the wall inlet. The pressure generated in the bottle was measured as the difference between the water levels 'A' and 'B'.
In order to standardize lung volume, we performed a LR manoeuvre and ventilated the patients with PEEP set at 1 cmH2O above LIP for 30 min before the ETS. This produced mean increases in lung volume and PaO2 of approximately 150 ml and 3 kPa, respectively. Interestingly, one patient (no. 2) changed lung injury category after the first standardization period from ARDS to ALI, and after the second standardization period that patient did not fulfil either the ARDS nor the ALI criteria of PaO2:FiO2 ratio [17]. Both lung volumes and compliance values may appear high for this type of patient but agree well with values found by Brochard and coworkers [13], who used computed tomography in patients with acute respiratory failure. Few studies have examined the reduction in lung volume caused by ETS. Brochard and coworkers [13] found that ETS caused an immediate reduction in EELV by about 400 ml in acute respiratory failure, and in patients with ALI and ARDS Pesenti and coworkers [10] identified a reduction in EELV by about 1200 ml. The different results might be due to differences in measurement techniques and patient populations, but not to differences in suction procedures because the pressures were similar (-80 and -100 mmHg, respectively), and the duration of suctioning was longer in the study by Brochard and coworkers. With our lung volume measurement technique it was not possible to measure EELV immediately but only after 5 min, at which time the reduction in EELV without a LR manoeuvre was about 200 ml, which is in accord with the study by Brochard and coworkers [13].
We found that a LR manoeuvre was effective as an additive measure to PEEP in rapidly regaining lung volume, compliance of the respiratory system and PaO2 after open ETS. However, just ventilation with PEEP did slowly increase both lung volume and PaO2. Because LR is an inspiratory phenomenon, the major effect of PEEP is prevention of de-recruitment of the lung regions recruited by the increased airway, or rather transpulmonary, pressure during inspiration. Even if we ventilated with small tidal volumes (i.e. about 7 ml/kg), this resulted in end-inspiratory airway pressures up to 35 cmH2O, which could very well have recruited some collapsed lung regions and improved oxygenation. However, higher end-inspiratory pressures are needed to recruit more manifest lung collapse [25,26]. This was indicated in the present study by the fact that, without a LR manoeuvre, the maximal compliance of the respiratory system obtained from the pressure–volume curves had not recovered at 25 min, and lung volume at all measurement points was lower as compared with ETS followed by a LR manoeuvre.
It is important to emphasize that a LR manoeuvre is not preventive, but rather is a therapeutic measure to regain lung volume and oxygenation rapidly after ETS. Four main methods have been suggested to prevent hypoxaemia in connection with ETS: administration of oxygen; hyperinflation of the lungs; closed-suction systems; and continuous flow insufflation. The former two methods are not very effective in patients with lungs that are prone to collapse and with high intrapulmonary shunt fractions, but may be used in less severe lung disease [6]. In addition, by using high inspired oxygen concentration, absorption atelectasis may develop [7]. The two latter methods might be effective in preventing hypoxaemia and lung collapse in ARDS [10,13,27,28]. However, with the closed suction system it is important that the ventilator is set at pressure-controlled or assisted mode, the trigger level is set low, and suction flow is less or similar to that delivered by the ventilator. Otherwise, a highly negative pressure may be generated, which is counterproductive [2,12]. Also, with continuous flow, the delivered flow should be higher than the flow in the suction catheter and sufficiently high to compensate for loss of air via the open endotracheal tube. In this context, it is also important to recognize the purpose of suctioning (i.e. removing secretions), and with a high bias flow this effect may be reduced. In lung-lavaged pigs, Lindgren and coworkers [11] showed that closed suctioning was less effective than open suctioning.
Nevertheless, the present study confirmed that ETS was associated with lung volume loss and hypoxaemia, and therefore we believe that preventive measures are important, and the most important measure is to avoid ETS at all if possible. In the present study, this is emphasized by the fact that, in one patient ventilated with FiO2 at 1.0, PaO2 decreased from 11 to 5 kPa during suctioning.
The study has some inherent limitations. First, the number of patients studied was low, although the number of patients was enough to ensure adequate significance between the procedures. Second, we studied only one kind of open suctioning procedure, and other procedures may give different results. Third, the patients were studied early in the disease process, and LR manoeuvres might have different effects in late ARDS. Fourth, the patients were haemodynamically stable and deeply sedated, and tolerated both ETS and the LR manoeuvres well and without circulatory compromise. In haemodynamically unstable or less deeply sedated patients, the results might be different. Finally, the LR manoeuvre was the same in all patients, and should preferably be individualized.
In conclusion, the present study confirms that open suctioning is associated with a substantial risk for hypoxaemia in patients with ARDS, stressing that ETS should be avoided unless absolutely necessary in such patients. Preferably, suction methods that prevent hypoxaemia should be used, but when open suctioning is indicated the present study suggests that a LR manoeuvre as an additive measure to PEEP causes a rapid recovery in EELV and PaO2.
Competing interests
None declared.
Key message
• LR manoeuvres, as additive measures to PEEP, are effective in reversing the reductions in EELV and PaO2 caused by ETS
Abbreviations
ALI = acute lung injury; ARDS = acute respiratory distress syndrome; EELV = end-expiratory lung volume; ETS = endotracheal suctioning; FiO2 = fractional inspired oxygen; LIP = lower inflection point; LR = lung recruitment; PaO2 = partial arterial oxygen tension; PEEP = positive end-expiratory pressure; SF6 = sulphur hexafluoride; SpO2 = blood oxygen saturation.
See related Commentary: http://ccforum.com/content/7/1/9
References
- Neumann P, Berglund JE, Mondejar EF, Magnusson A, Hedenstierna G. Dynamics of lung collapse and recruitment during prolonged breathing in porcine lung injury. J Appl Physiol. 1998;85:1533–1543. doi: 10.1152/jappl.1998.85.4.1533. [DOI] [PubMed] [Google Scholar]
- Stenqvist O, Lindgren S, Karason S, Sondergaard S, Lundin S. Warning! Suctioning. A lung model evaluation of closed suctioning systems. Acta Anaesthesiol Scand. 2001;45:167–172. doi: 10.1034/j.1399-6576.2001.450206.x. [DOI] [PubMed] [Google Scholar]
- Clark AP, Winslow EH, Tyler DO, White KM. Effects of endotracheal suctioning on mixed venous oxygen saturation and heart rate in critically ill adults. Heart Lung. 1990;19:552–557. [PubMed] [Google Scholar]
- Grap MJ, Glass C, Corley M, Parks T. Endotracheal suctioning: ventilator vs manual delivery of hyperoxygenation breaths. Am J Crit Care. 1996;5:192–197. [PubMed] [Google Scholar]
- Preusser BA, Stone KS, Gonyon DS, Winningham ML, Groch KF, Karl JE. Effects of two methods of preoxygenation on mean arterial pressure, cardiac output, peak airway pressure, and postsuctioning hypoxemia. Heart Lung. 1988;17:290–299. [PubMed] [Google Scholar]
- Lumb A. Distribution of pulmonary ventilation and perfusion. In: Lumb A, editor. In Nunn's Applied Respiratory Physiology. 5. Oxford: Butterworth-Heinemann; 2000. pp. 163–199. [Google Scholar]
- Santos C, Ferrer M, Roca J, Torres A, Hernandez C, Rodriguez-Roisin R. Pulmonary gas exchange response to oxygen breathing in acute lung injury. Am J Respir Crit Care Med. 2000;161:26–31. doi: 10.1164/ajrccm.161.1.9902084. [DOI] [PubMed] [Google Scholar]
- Rothen HU, Sporre B, Engberg G, Wegenius G, Reber A, Hedenstierna G. Prevention of atelectasis during general anaesthesia. Lancet. 1995;345:1387–1391. doi: 10.1016/s0140-6736(95)92595-3. [DOI] [PubMed] [Google Scholar]
- Rothen HU, Sporre B, Engberg G, Wegenius G, Hogman M, Hedenstierna G. Influence of gas composition on recurrence of atelectasis after a reexpansion maneuver during general anesthesia. Anesthesiology. 1995;82:832–842. doi: 10.1097/00000542-199504000-00004. [DOI] [PubMed] [Google Scholar]
- Cereda M, Villa F, Colombo E, Greco G, Nacoti M, Pesenti A. Closed system endotracheal suctioning maintains lung volume during volume-controlled mechanical ventilation. Intensive Care Med. 2001;27:648–654. doi: 10.1007/s001340100897. [DOI] [PubMed] [Google Scholar]
- Lindgren S, Almgren B, Högman M, Lethvall S, Lundin S, Stenqvist O. Closed system suctioning has little suctioning effect and little side-effects [abstract]. Intensive Care Med. 2001;27(suppl 2):S246. doi: 10.1007/s00134-003-2153-9. [DOI] [PubMed] [Google Scholar]
- Taggart JA, Dorinsky NL, Sheahan JS. Airway pressures during closed system suctioning. Heart Lung. 1988;17:536–542. [PubMed] [Google Scholar]
- Brochard L, Mion G, Isabey D, Bertrand C, Messadi AA, Mancebo J, Boussignac G, Vasile N, Lemaire F, Harf A. Constant-flow insufflation prevents arterial oxygen desaturation during endotracheal suctioning. Am Rev Respir Dis. 1991;144:395–400. doi: 10.1164/ajrccm/144.2.395. [DOI] [PubMed] [Google Scholar]
- Goodnough SK. The effects of oxygen and hyperinflation on arterial oxygen tension after endotracheal suctioning. Heart Lung. 1985;14:11–17. [PubMed] [Google Scholar]
- Stone KS, Vorst EC, Lanham B, Zahn S. Effects of lung hyperinflation on mean arterial pressure and postsuctioning hypoxemia. Heart Lung. 1989;18:377–385. [PubMed] [Google Scholar]
- Lu Q, Capderou A, Cluzel P, Mourgeon E, Abdennour L, Law-Koune JD, Straus C, Grenier P, Zelter M, Rouby JJ. A computed tomographic scan assessment of endotracheal suctioning-induced bronchoconstriction in ventilated sheep. Am J Respir Crit Care Med. 2000;162:1898–1904. doi: 10.1164/ajrccm.162.5.2003105. [DOI] [PubMed] [Google Scholar]
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. Report of the American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. The Consensus Committee. Intensive Care Med. 1994;20:225–232. doi: 10.1007/BF01704707. [DOI] [PubMed] [Google Scholar]
- Lapinsky SE, Aubin M, Mehta S, Boiteau P, Slutsky AS. Safety and efficacy of a sustained inflation for alveolar recruitment in adults with respiratory failure. Intensive Care Med. 1999;25:1297–1301. doi: 10.1007/s001340050104. [DOI] [PubMed] [Google Scholar]
- Larsson A, Linnarsson D, Jonmarker C, Jonson B, Larsson H, Werner O. Measurement of lung volume by sulfur hexafluoride washout during spontaneous and controlled ventilation: further development of a method. Anesthesiology. 1987;67:543–550. doi: 10.1097/00000542-198710000-00016. [DOI] [PubMed] [Google Scholar]
- Svantesson C, Drefeldt B, Sigurdsson S, Larsson A, Brochard L, Jonson B. A single computer-controlled mechanical insufflation allows determination of the pressure-volume relationship of the respiratory system. J Clin Monit Comput. 1999;15:9–16. doi: 10.1023/A:1009916905078. [DOI] [PubMed] [Google Scholar]
- Jonson B, Richard JC, Straus C, Mancebo J, Lemaire F, Brochard L. Pressure–volume curves and compliance in acute lung injury: evidence of recruitment above the lower inflection point. Am J Respir Crit Care Med. 1999;159:1172–1178. doi: 10.1164/ajrccm.159.4.9801088. [DOI] [PubMed] [Google Scholar]
- Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- Berman IR, Stahl WM. Prevention of hypoxic complications during endotracheal suctioning. Surgery. 1968;63:586–587. [PubMed] [Google Scholar]
- Bodai BI. A means of suctioning without cardiopulmonary depression. Heart Lung. 1982;11:172–176. [PubMed] [Google Scholar]
- Medoff BD, Harris RS, Kesselman H, Venegas J, Amato MB, Hess D. Use of recruitment maneuvers and high-positive end-expiratory pressure in a patient with acute respiratory distress syndrome. Crit Care Med. 2000;28:1210–1216. doi: 10.1097/00003246-200004000-00051. [DOI] [PubMed] [Google Scholar]
- Pelosi P, Cadringher P, Bottino N, Panigada M, Carrieri F, Riva E, Lissoni A, Gattinoni L. Sigh in acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;159:872–880. doi: 10.1164/ajrccm.159.3.9802090. [DOI] [PubMed] [Google Scholar]
- Kelly RE, Yao FS, Artusio JF., Jr Prevention of suction-induced hypoxemia by simultaneous oxygen insufflation. Crit Care Med. 1987;15:874–875. doi: 10.1097/00003246-198709000-00016. [DOI] [PubMed] [Google Scholar]
- Bodai BI, Walton CB, Briggs S, Goldstein M. A clinical evaluation of an oxygen insufflation/suction catheter. Heart Lung. 1987;16:39–46. [PubMed] [Google Scholar]


