Abstract
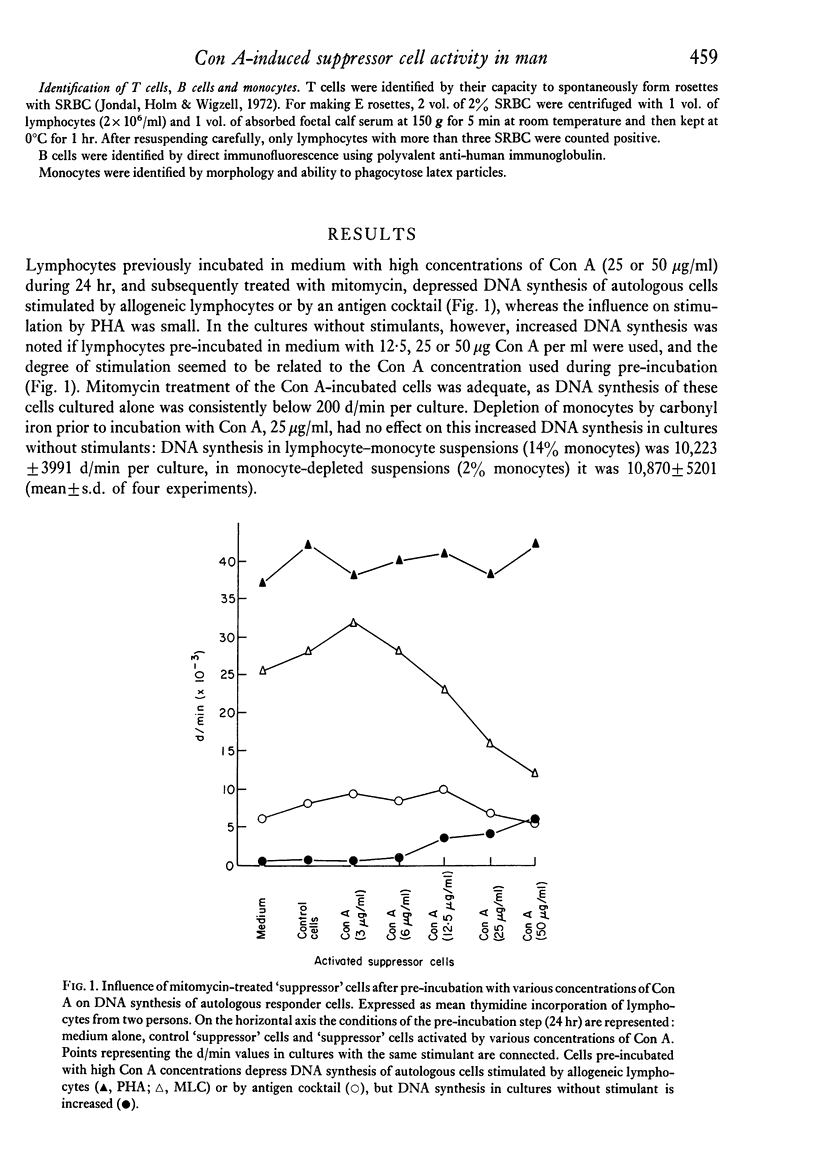
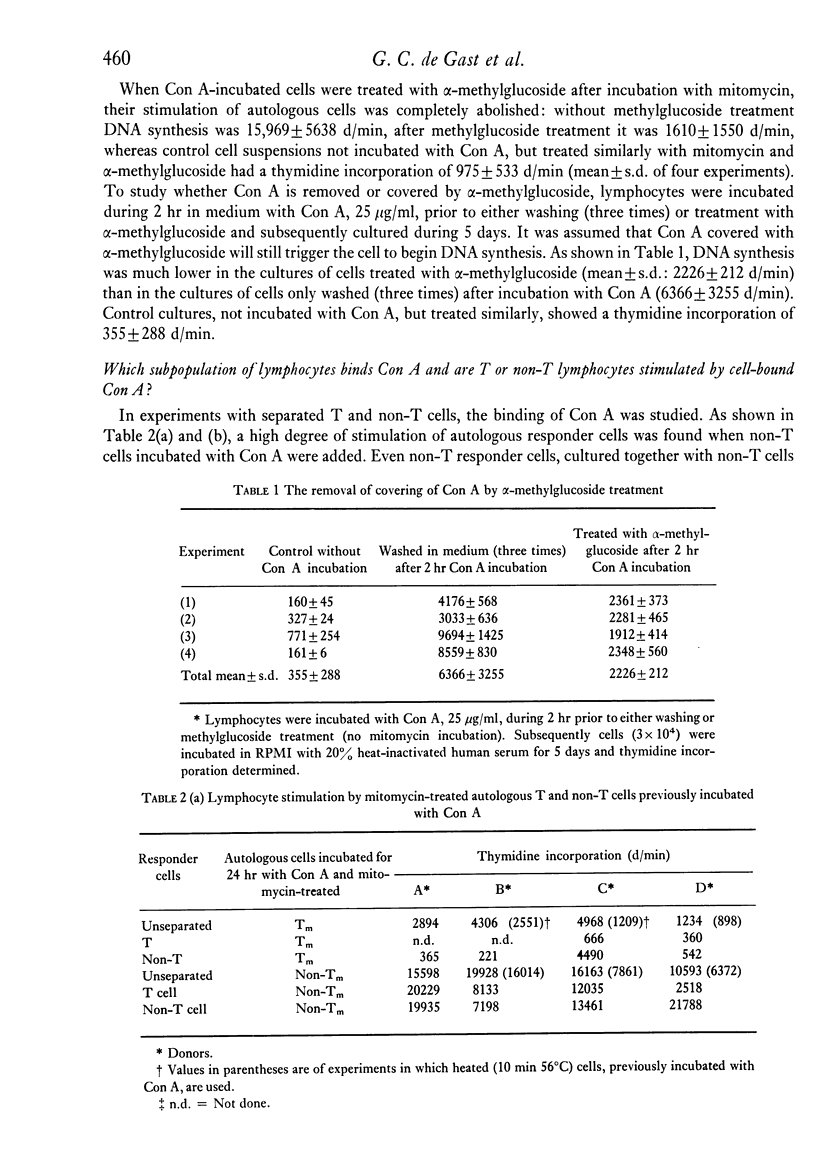
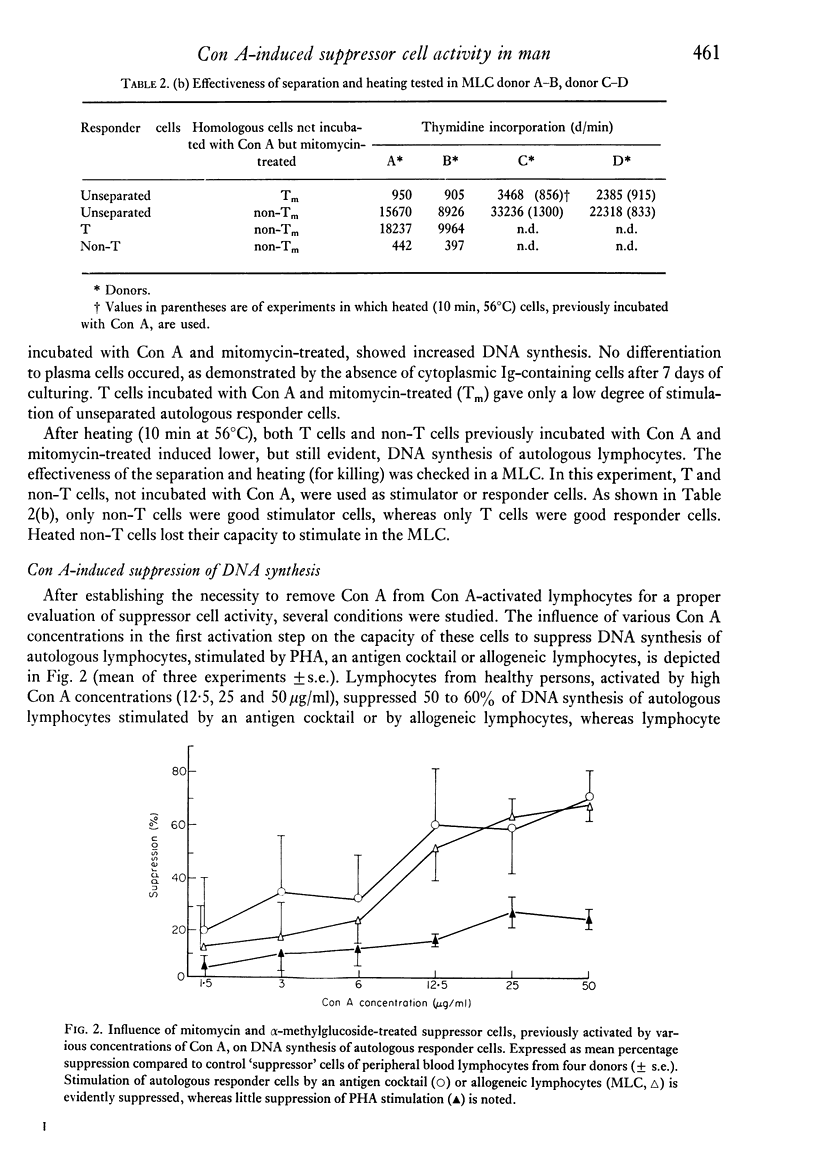
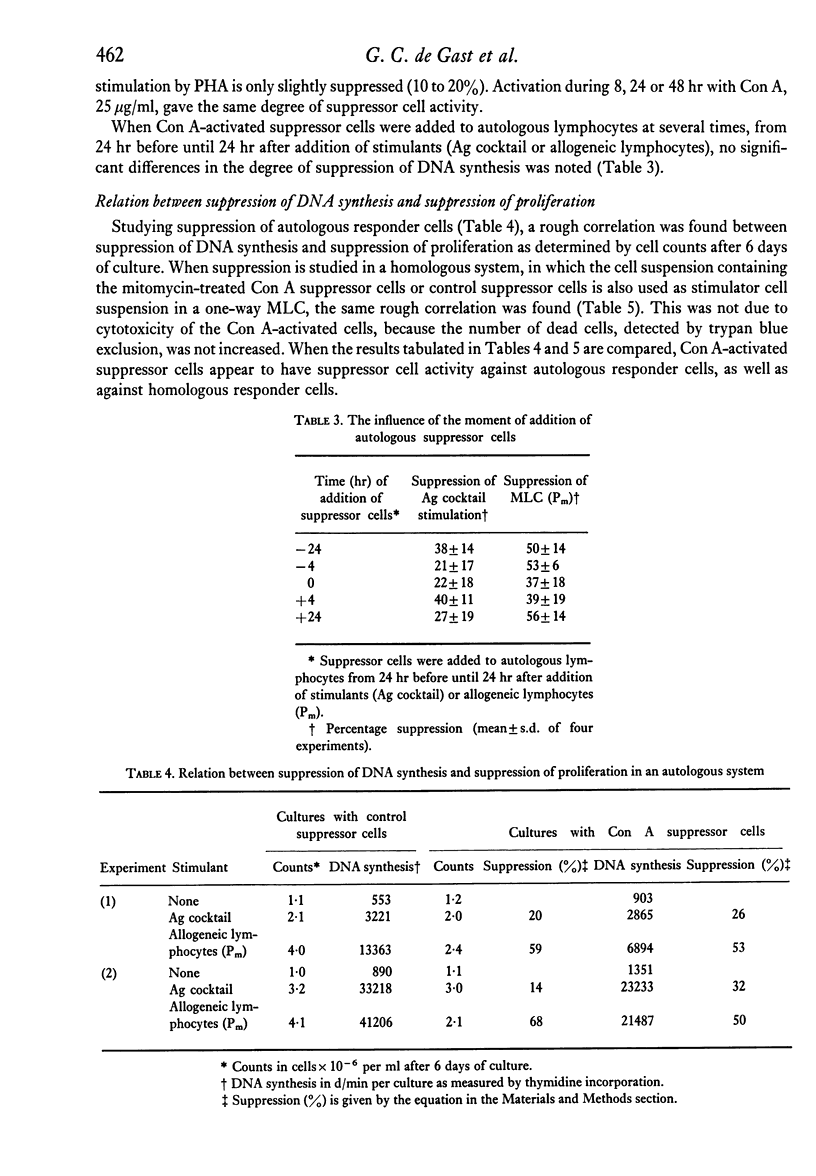
The capacity of human peripheral blood lymphocytes to suppress DNA synthesis of other lymphocytes was studied in an assay consisting of two steps: firstly, activation by Con A during 24 hr followed by alpha-methylglucoside and mitomycin treatment; secondly, incubation of these Con A-activated 'suppressor' cells with autologous responder cells and stimulants, or incubation with allogeneic responder cells. The results were compared with cells similarly treated but not incubated with Con A. If alpha-methylglucoside treatment is omitted, stimulation of T and non-T cells occurs by Con A bound to the Con A-activated cells. Con A is especially bound to non-T lymphocytes and even gives a T cell-independent proliferation of non-T cells without differentiation to plasma cells. With alpha-methylglucoside treatment, 'suppressor' cells, activated by high Con A concentrations, are able to suppress DNA synthesis of autologous lymphocytes stimulated by allogeneic cells or soluble antigens to about 50%. In a one-way MLC, in which the cell suspension containing the suppressor cells is also used as a stimulator cell suspension, a similar suppression was observed. Suppression of DNA synthesis was correlated with suppression of proliferation without evidence of cytotoxicity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Edelman G. M., Möller G., Sjöberg O. Activation of B lymphocytes by locally concentrated concanavalin A. Eur J Immunol. 1972 Jun;2(3):233–235. doi: 10.1002/eji.1830020307. [DOI] [PubMed] [Google Scholar]
- Asherson G. L., Ferluga J., Janossy G. Non-specific cytotoxicity by T cells activated with plant mitogens in vitro and the requirement for plant agents during the killing reaction. Clin Exp Immunol. 1973 Dec;15(4):573–589. [PMC free article] [PubMed] [Google Scholar]
- Broder S., Humphrey R., Durm M., Blackman M., Meade B., Goldman C., Strober W., Waldmann T. Impaired synthesis of polyclonal (non-paraprotein) immunoglobulins by circulating lymphocytes from patients with multiple myeloma Role of suppressor cells. N Engl J Med. 1975 Oct 30;293(18):887–892. doi: 10.1056/NEJM197510302931801. [DOI] [PubMed] [Google Scholar]
- De Gast G. C., Houwen B., van der Hem G. K., The T. H. T-lymphocyte number and function and the course of hepatitis B in hemodialysis patients. Infect Immun. 1976 Nov;14(5):1138–1143. doi: 10.1128/iai.14.5.1138-1143.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton R. W. Inhibitory and stimulatory effects of concanavalin A on the response of mouse spleen cell suspensions to antigen. I. Characterization of the inhibitory cell activity. J Exp Med. 1972 Dec 1;136(6):1445–1460. doi: 10.1084/jem.136.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch H., Waksman B. H. Regulation of lymphocyte responses in vitro. V. Suppressor activity of adherent and nonadherent rat lymphoid cells. Cell Immunol. 1973 Oct;9(1):12–24. doi: 10.1016/0008-8749(73)90163-9. [DOI] [PubMed] [Google Scholar]
- Folch H., Waksman B. H. The splenic suppressor cell. II. Suppression of mixed lymphocyte reaction by thymus-dependent adherent cells. J Immunol. 1974 Jul;113(1):140–144. [PubMed] [Google Scholar]
- Greaves M. F., Bauminger S., Janossy G. Lymphocyte activation. 3. Binding sites for phytomitogens on lymphocyte subpopulations. Clin Exp Immunol. 1972 Mar;10(3):537–554. [PMC free article] [PubMed] [Google Scholar]
- Hoffman R., Zanjani E. D., Lutton J. D., Zalusky R., Wasserman L. R. Suppression of erythroid-colony formation by lymphocytes from patients with aplastic anemia. N Engl J Med. 1977 Jan 6;296(1):10–13. doi: 10.1056/NEJM197701062960103. [DOI] [PubMed] [Google Scholar]
- Hubert C., Delespesse G., Govaerts A. Concanavalin A-activated suppressor cells in normal human peripheral blood lymphocytes. Clin Exp Immunol. 1976 Oct;26(1):95–98. [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller G., Andersson J., Pohlit H., Sjöberg O. Quantitation of the number of mitogen molecules activating DNA synthesis in T and B lymphocytes. Clin Exp Immunol. 1973 Jan;13(1):89–99. [PMC free article] [PubMed] [Google Scholar]
- Peavy D. L., Pierce C. W. Cell-mediated immune responses in vitro. I. Suppression of the generation of cytotoxic lymphocytes by concanavalin A and concanavalin A-activated spleen cells. J Exp Med. 1974 Aug 1;140(2):356–369. doi: 10.1084/jem.140.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak L., Geleick H. Differing mechanisms of tolerance and desensitization to dinitrochlorobenzene in guinea pigs. Eur J Immunol. 1975 Feb;5(2):94–99. doi: 10.1002/eji.1830050205. [DOI] [PubMed] [Google Scholar]
- Rich R. R., Pierce C. W. Biological expressions of lymphocyte activation. II. Generation of a population of thymus-derived suppressor lymphocytes. J Exp Med. 1973 Mar 1;137(3):649–659. doi: 10.1084/jem.137.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich R. R., Rich S. S. Biological expressions of lymphocyte activation. IV. Concanavalin A-activated suppressor cells in mouse mixed lymphocyte reactions. J Immunol. 1975 Mar;114(3):1112–1115. [PubMed] [Google Scholar]
- Sampson D., Grotelueschen C., Kauffman H. M., Jr The human splenic suppressor cell. Transplantation. 1975 Nov;20(5):362–367. doi: 10.1097/00007890-197511000-00002. [DOI] [PubMed] [Google Scholar]
- Shou L., Schwartz S. A., Good R. A. Suppressor cell activity after concanavalin A treatment of lymphocytes from normal donors. J Exp Med. 1976 May 1;143(5):1100–1110. doi: 10.1084/jem.143.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twomey J. J., Laughter A. H., Farrow S., Douglass C. C. Hodgkin's disease. An immunodepleting and immunosuppressive disorder. J Clin Invest. 1975 Aug;56(2):467–475. doi: 10.1172/JCI108113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A., Durm M., Broder S., Blackman M., Blaese R. M., Strober W. Role of suppressor T cells in pathogenesis of common variable hypogammaglobulinaemia. Lancet. 1974 Sep 14;2(7881):609–613. doi: 10.1016/s0140-6736(74)91940-0. [DOI] [PubMed] [Google Scholar]
- Weksler M. E. Lymphocyte transformation induced by autologous cells. III. Lymphoblast-induced lymphocyte to stimulation does not correlate with EB viral antigen expression or immunity. J Immunol. 1976 Feb;116(2):310–314. [PubMed] [Google Scholar]
- Zembala M., Asherson G. L. Depression of the T cell phenomenon of contact sensitivity by T cells from unresponsive mice. Nature. 1973 Jul 27;244(5413):227–228. doi: 10.1038/244227a0. [DOI] [PubMed] [Google Scholar]


