Abstract
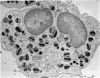
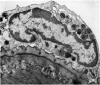
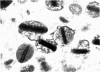
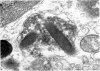
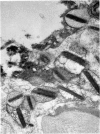
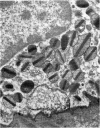
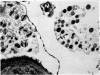
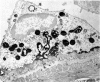
Rat eosinophils form an intimate association with the surfaces of parasitic helminths, in vitro, in the presence of immune serum. The parasite presents a non-phagocytosable surface to the cell. The initial response of the eosinophil is degranulation which leads to the formation of large cytoplasmic vacuoles. Peroxidase, an enzyme localized in the matrix of the crystalloid secretion granules, is discharged into these vacuoles as a consequence of degranulation. The vacuoles eventually become connected to the adherent basal plasma membrane of the eosinophil, and peroxidase is secreted directly onto the surface of the parasite. There is no morphological evidence to suggest that this particular secretion affects the integrity of the parasite surface.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARCHER G. T., HIRSCH J. G. MOTION PICTURE STUDIES ON DEGRANULATION OF HORSE EOSINOPHILS DURING PHAGOCYTOSIS. J Exp Med. 1963 Aug 1;118:287–294. doi: 10.1084/jem.118.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer G. T., Nelson M., Johnston J. Eosinophil granule lysis in vitro induced by soluble antigen-antibody complexes. Immunology. 1969 Nov;17(5):777–787. [PMC free article] [PubMed] [Google Scholar]
- Butterworth A. E., David J. R., Franks D., Mahmoud A. A., David P. H., Sturrock R. F., Houba V. Antibody-dependent eosinophil-mediated damage to 51Cr-labeled schistosomula of Schistosoma mansoni: damage by purieid eosinophils. J Exp Med. 1977 Jan 1;145(1):136–150. doi: 10.1084/jem.145.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotran R. S., Litt M. The entry of granule-associated peroxidase into the phagocytic vacuoles of eosinophils. J Exp Med. 1969 Jun 1;129(6):1291–1306. doi: 10.1084/jem.129.6.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. I. The role of antibody and complement on nonphagocytosable surfaces or phagocytosable particles. J Immunol. 1971 Dec;107(6):1535–1546. [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. II. Mechanisms of release during phagocytosis, and adherence to nonphagocytosable surfaces. J Immunol. 1971 Dec;107(6):1547–1557. [PubMed] [Google Scholar]
- Hudson G. Eosinophil granules and cell maturity: electron microscopic observations on guinea-pig marrow. Acta Haematol. 1966;36(5):350–360. doi: 10.1159/000209416. [DOI] [PubMed] [Google Scholar]
- Hudson G. Eosinophil granules and phosphotungstic acid: an electron microscope study of guinea-pig bone marrow. Exp Cell Res. 1966 Feb;41(2):265–273. doi: 10.1016/s0014-4827(66)80134-9. [DOI] [PubMed] [Google Scholar]
- Hudson G. Eosinophil granules and uranyl acetate. An electron microscope study of guinea-pig bone marrow. Exp Cell Res. 1967 Apr;46(1):121–128. doi: 10.1016/0014-4827(67)90414-4. [DOI] [PubMed] [Google Scholar]
- Hudson G., Heap P. Ultrastructure of the eosinophil granule-internum; the problem of reversed relative density. Z Zellforsch Mikrosk Anat. 1969;93(3):332–335. doi: 10.1007/BF00332660. [DOI] [PubMed] [Google Scholar]
- Kelényi G., Zombai E., Németh A. Histochemische und elektronenmikroskopische Beobachtungen an der spezifischen Granulation der eosinophilen Granulozyten. Acta Histochem. 1965 Sep 25;22(1):77–89. [PubMed] [Google Scholar]
- Lewis P. R., Shute C. C. The distribution of cholinesterase in cholinergic neurons demonstrated with the electron microscope. J Cell Sci. 1966 Sep;1(3):381–390. doi: 10.1242/jcs.1.3.381. [DOI] [PubMed] [Google Scholar]
- Love R. J., Ogilvie B. M., McLaren D. J. The immune mechanism which expels the intestinal stage of Trichinella spiralis from rats. Immunology. 1976 Jan;30(1):7–15. [PMC free article] [PubMed] [Google Scholar]
- Love R. J., Ogilvie B. M. Nippostrongylus brasiliensis in young rats. Lymphocytes expel larval infections but not adult worms. Clin Exp Immunol. 1975 Jul;21(1):155–162. [PMC free article] [PubMed] [Google Scholar]
- McLaren D. J. The anterior glands of adult Necator americanus (Nematoda: Strongyloidea). I. Ultrastructural studies. Int J Parasitol. 1974 Feb;4(1):25–37. doi: 10.1016/0020-7519(74)90006-x. [DOI] [PubMed] [Google Scholar]
- Ramalho-Pinto F. J., Goldring O. L., Smithers S. R., Playfair H. L. T-cell helper response to antigens of Schistosoma mansoni in CBA mice. Clin Exp Immunol. 1976 Nov;26(2):327–333. [PMC free article] [PubMed] [Google Scholar]
- Skinnider L. F., Ghadially F. N. Secretion of granule content by eosinophils. Arch Pathol. 1974 Jul;98(1):58–61. [PubMed] [Google Scholar]
- Smithers S. R., Terry R. J. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology. 1965 Nov;55(4):695–700. doi: 10.1017/s0031182000086248. [DOI] [PubMed] [Google Scholar]
- Spry C. J., Tai P. C. Studies on blood eosinophils. II. Patients with Löffler's cardiomyopathy. Clin Exp Immunol. 1976 Jun;24(3):423–434. [PMC free article] [PubMed] [Google Scholar]
- Tai P. C., Spry C. J. Studies on blood eosinophils. I. Patients with a transient eosinophilia. Clin Exp Immunol. 1976 Jun;24(3):415–422. [PMC free article] [PubMed] [Google Scholar]
- Yamada E., Yamauchi R. [Some observations on the cytochemistry and morphogenesis of the granulocytes in the rat bone marrow as revealed by electron microscopy]. Nihon Ketsueki Gakkai Zasshi. 1966 Aug;29(4):530–541. [PubMed] [Google Scholar]