Abstract
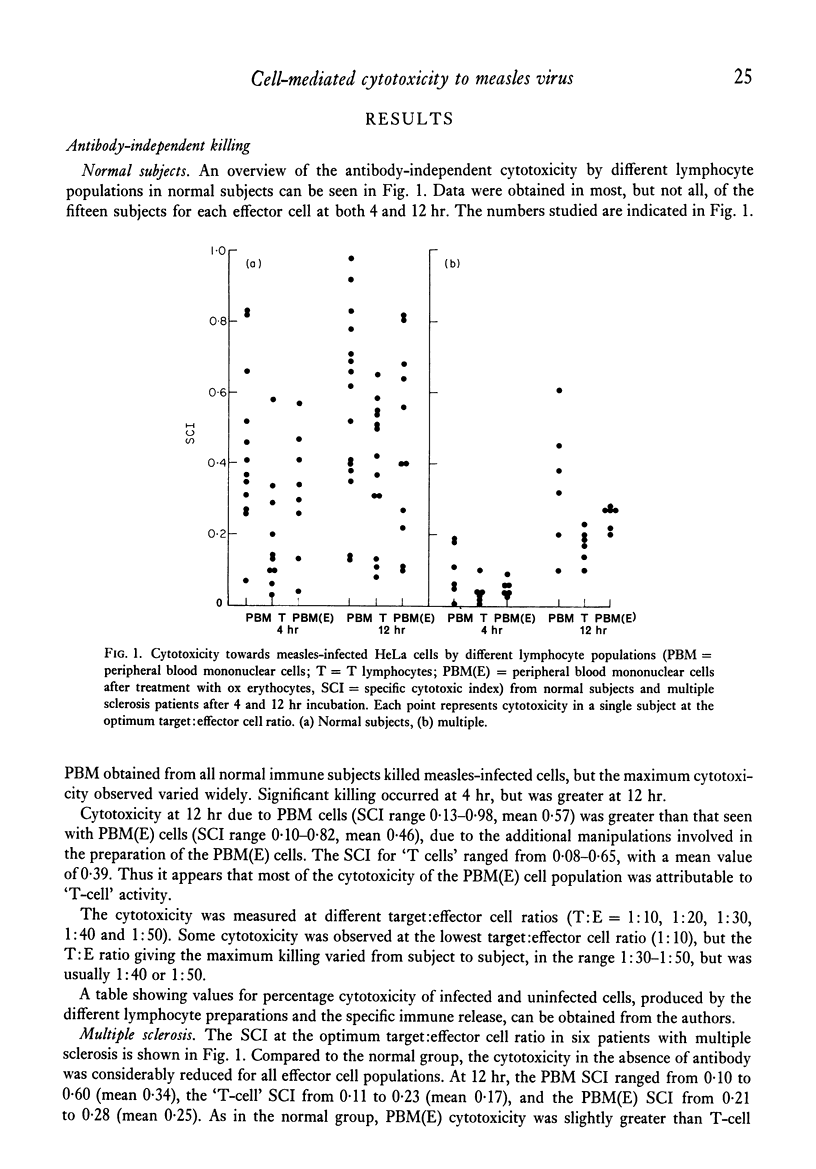
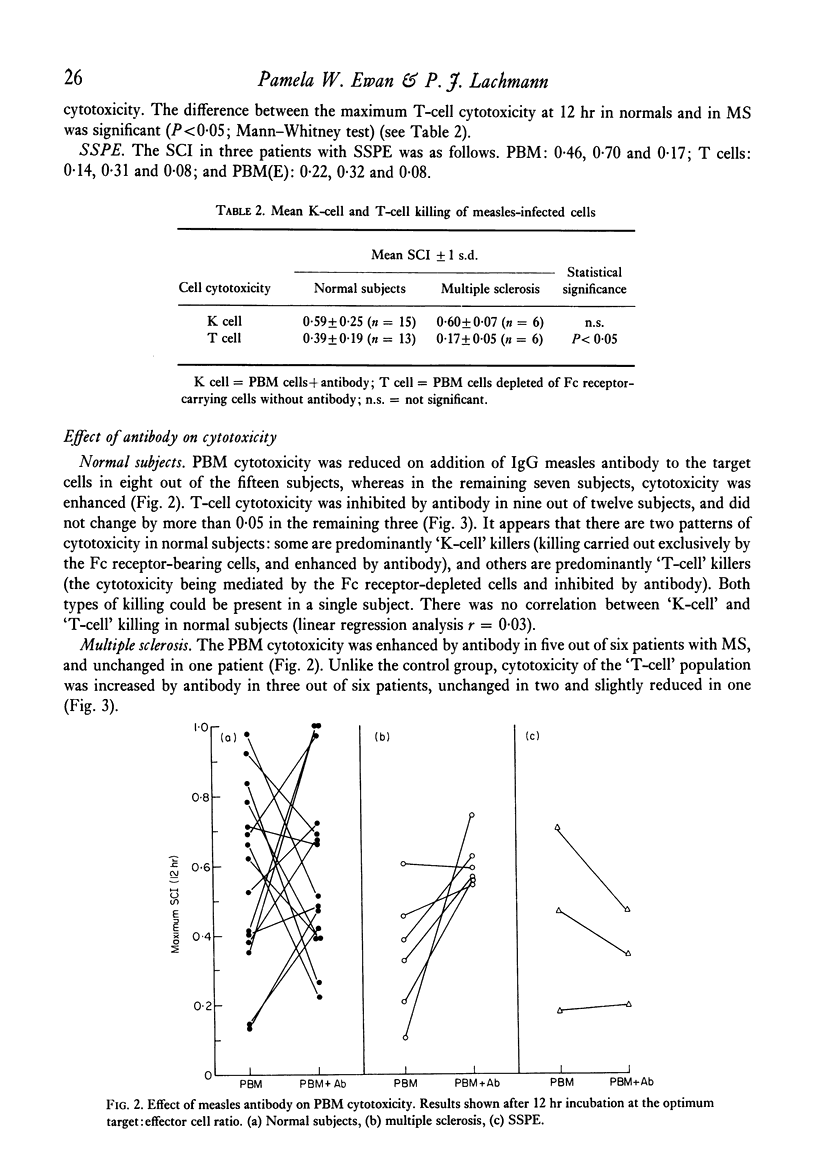
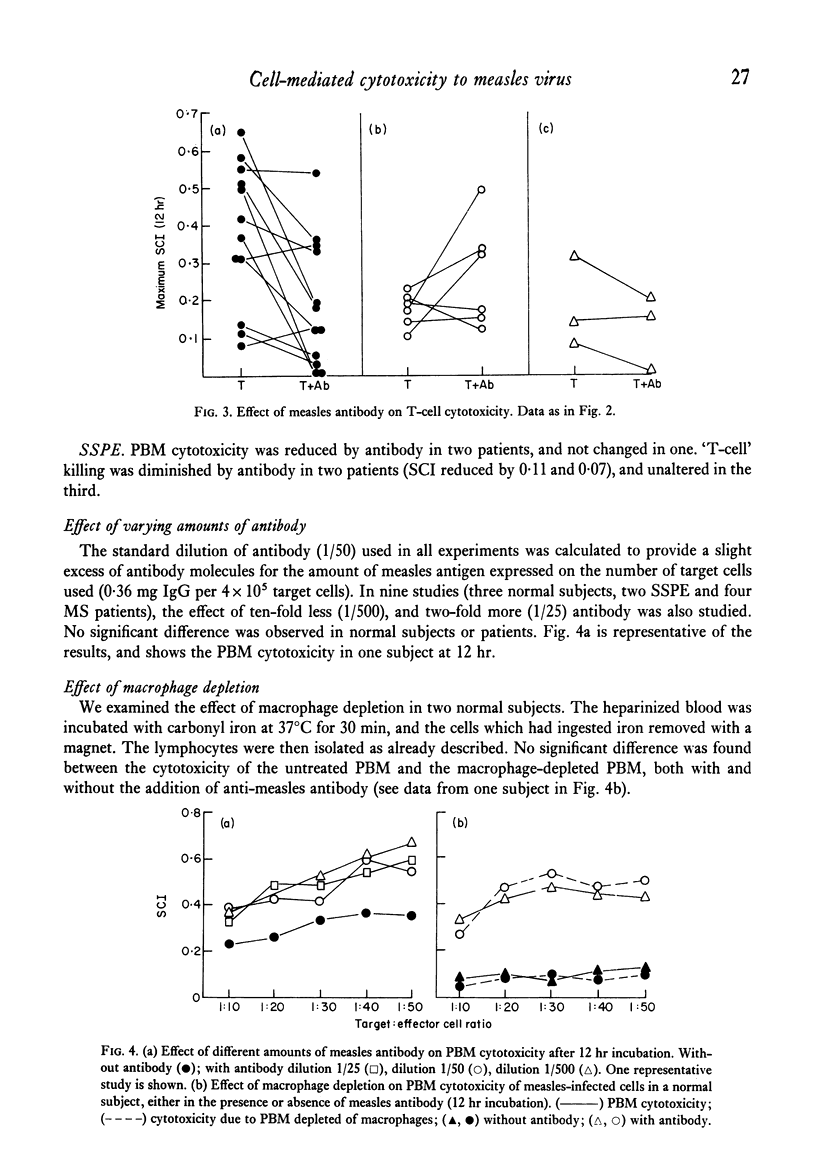
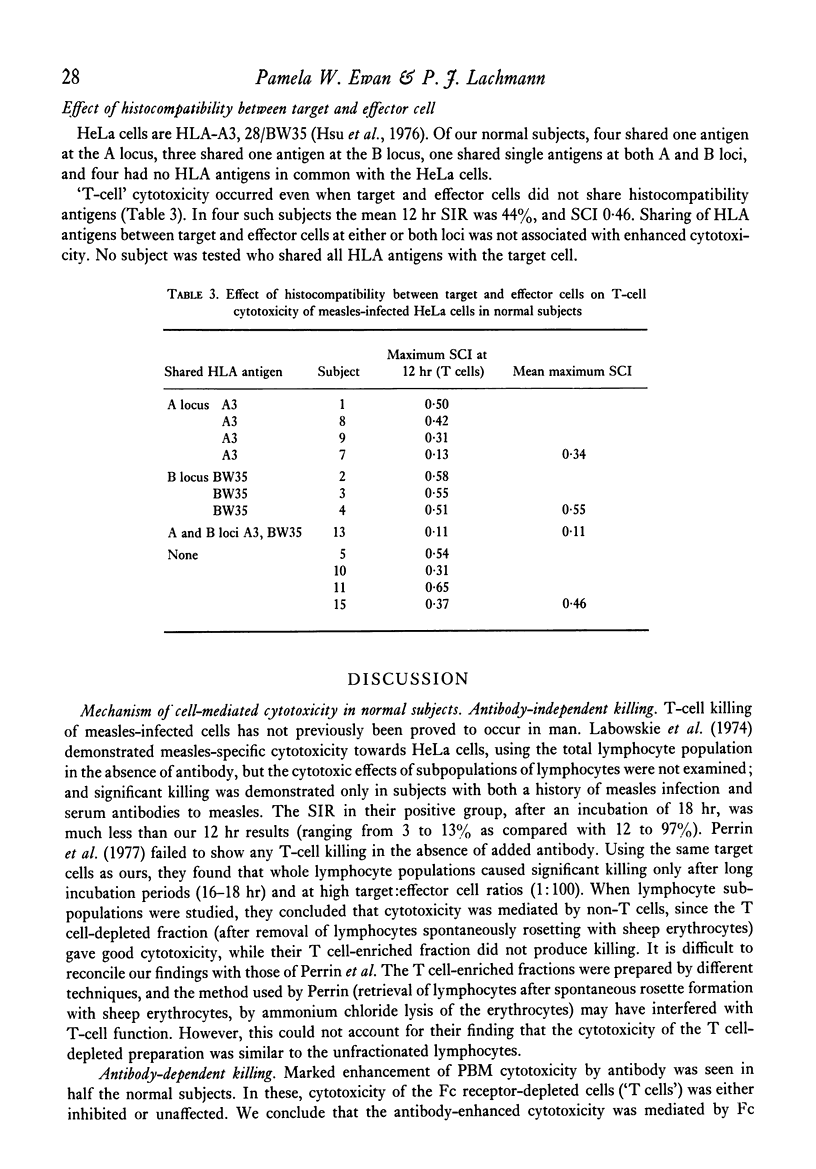
Mechanisms of cell-mediated cytotoxicity towards a human cell line persistently infected with measles virus were studied in fifteen normal (measles-immune) subjects, six patients with multiple sclerosis and three patients with subacute sclerosing panencephalitis. In normal subjects, cytotoxicity by peripheral blood mononuclear cells (PBM) in the absence of measles antibody was demonstrated, and shown by lymphocyte fractionation (removal of Fc receptor-bearing cells) to be T cell-mediated. The mean T cell-specific cytotoxic index after 12 hr incubation at the optimum target:effector cell ratio was 0·39±0·19 (1 s.d.). T-cell killing was inhibited by antibody in nine out of twelve subjects, and not significantly changed in three. Antibody enhancement of killing by PBM was found in seven out of fifteen normal subjects, but antibody did not enhance killing by a cell population depleted of Fc receptor-bearing cells. We therefore conclude that the antibody-dependent killing was mediated by Fc receptor-bearing cells (K cells). Mean cytotoxicity due to PBM in the presence of antibody at 12 hr was 0·59±0·25 (1 s.d.). Patients with multiple sclerosis showed significantly impaired T-cell cytotoxicity (mean ± 1 s.d. = 0·17±0·05), although there was overlap with the normal group, while PBM killing in the presence of antibody was normal (mean ± 1 s.d. = 0·60±0·07). Normal or low values for both types of cytotoxicity were found in three patients with subacute sclerosing panencephalitis. Contrary to evidence for several other viruses in mice, showing H-2 restriction of T-cell killing, we have demonstrated good T-cell cytotoxicity independent of shared HLA antigens between target and effector cell for measles virus infection.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS J. M., IMAGAWA D. T. Measles antibodies in multiple sclerosis. Proc Soc Exp Biol Med. 1962 Dec;111:562–566. doi: 10.3181/00379727-111-27855. [DOI] [PubMed] [Google Scholar]
- Ahmed A., Strong D. M., Sell K. W., Thurman G. B., Knudsen R. C., Wistar R., Jr, Grace W. R. Demonstration of a blocking factor in the plasma and spinal fluid of patients with subacute sclerosing panencephalitis. I. Partial characterization. J Exp Med. 1974 Apr 1;139(4):902–924. doi: 10.1084/jem.139.4.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson T., Stejskal V., Harfast B. An in vitro method for study of human lymphocyte cytotoxicity against mumps-virus-infected target cells. J Immunol. 1975 Jan;114(1 Pt 1):237–243. [PubMed] [Google Scholar]
- Blanden R. V., Doherty P. C., Dunlop M. B., Gardner I. D., Zinkernagel R. M., David C. S. Genes required for cytotoxicity against virus-infected target cells in K and D regions of H-2 complex. Nature. 1975 Mar 20;254(5497):269–270. doi: 10.1038/254269a0. [DOI] [PubMed] [Google Scholar]
- Brunner K. T., Mauel J., Cerottini J. C., Chapuis B. Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology. 1968 Feb;14(2):181–196. [PMC free article] [PubMed] [Google Scholar]
- Burnet F. M. Measles as an index of immunological function. Lancet. 1968 Sep 14;2(7568):610–613. doi: 10.1016/s0140-6736(68)90701-0. [DOI] [PubMed] [Google Scholar]
- Connolly J. H., Allen I. V., Hurwitz L. J., Millar J. H. Measles-virus antibody and antigen in subacute sclerosing panencephalitis. Lancet. 1967 Mar 11;1(7489):542–544. doi: 10.1016/s0140-6736(67)92117-4. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Zinernagel R. M. Capacity of sensitized thymus-derived lymphocytes to induce fatal lymphocytic choriomeningitis is restricted by the H-2 gene complex. J Immunol. 1975 Jan;114(1 Pt 1):30–33. [PubMed] [Google Scholar]
- Doherty P. C., Zinkernagel R. M. A biological role for the major histocompatibility antigens. Lancet. 1975 Jun 28;1(7922):1406–1409. doi: 10.1016/s0140-6736(75)92610-0. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Zinkernagel R. M. H-2 compatibility is required for T-cell-mediated lysis of target cells infected with lymphocytic choriomeningitis virus. J Exp Med. 1975 Feb 1;141(2):502–507. doi: 10.1084/jem.141.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C., Zinkernagel R. M. Specific immune lysis of paramyxovirus-infected cells by H-2-compatible thymus-derived lymphocytes. Immunology. 1976 Jul;31(1):27–32. [PMC free article] [PubMed] [Google Scholar]
- Freeman J. M., Magoffin R. L., Lennette E. H., Herndon R. M. Additional evidence of the relation between subacute inclusion-body encephalitis and measles virus. Lancet. 1967 Jul 15;2(7507):129–131. doi: 10.1016/s0140-6736(67)92965-0. [DOI] [PubMed] [Google Scholar]
- Haire M., Fraser K. B., Millar J. H. Virus-specific immunoglobulins in multiple sclerosis. Clin Exp Immunol. 1973 Jul;14(3):409–416. [PMC free article] [PubMed] [Google Scholar]
- Horta-Barbosa L., Fuccillo D. A., Sever J. L., Zeman W. Subacute sclerosing panencephalitis: isolation of measles virus from a brain biopsy. Nature. 1969 Mar 8;221(5184):974–974. doi: 10.1038/221974a0. [DOI] [PubMed] [Google Scholar]
- Hsu S. H., Schacter B. Z., Delaney N. L., Miller T. B., McKusick V. A., Kennett R. H., Bodmer J. G., Young D., Bodmer W. F. Genetic characteristics of the HeLa cell. Science. 1976 Jan 30;191(4225):392–394. doi: 10.1126/science.1246620. [DOI] [PubMed] [Google Scholar]
- Härfast B., Andersson T., Perlmann P. Human lymphocyte cytotoxicity against mumps virus-infected target cells. Requirement for non-T cells. J Immunol. 1975 Jun;114(6):1820–1823. [PubMed] [Google Scholar]
- Jabbour J. T., Roane J. A., Sever J. L. Studies of delayed dermal hypersensitivity in patients with subacute sclerosing panencephalitis. Neurology. 1969 Oct;19(10):929–931. doi: 10.1212/wnl.19.10.929. [DOI] [PubMed] [Google Scholar]
- Joseph B. S., Oldstone M. B. Antibody-induced redistribution of measles virus antigens on the cell surface. J Immunol. 1974 Oct;113(4):1205–1209. [PubMed] [Google Scholar]
- Joseph B. S., Oldstone M. B. Immunologic injury in measles virus infection. II. Suppression of immune injury through antigenic modulation. J Exp Med. 1975 Oct 1;142(4):864–876. doi: 10.1084/jem.142.4.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszinowski U., Ertl H. Lysis mediated by T cells and restricted by H-2 antigen of target cells infected with vaccinia virus. Nature. 1975 Jun 12;255(5509):552–554. doi: 10.1038/255552a0. [DOI] [PubMed] [Google Scholar]
- Kreth H. W., Meulen V. Cell-mediated cytotoxicity against measles virus in SSPE. I. Enhancement by antibody. J Immunol. 1977 Jan;118(1):291–295. [PubMed] [Google Scholar]
- Kreth H. W., Wiegand G. Cell-mediated cytotoxicity against measles virus in SSPE. II. Analysis of cytotoxic effector cells. J Immunol. 1977 Jan;118(1):296–301. [PubMed] [Google Scholar]
- Labowskie R., Edelman R., Rustigian R., Bellanti J. A. Studies of cell-mediated immunity to measles virus by in vitro lymphocyte-mediated cytotoxicity. J Infect Dis. 1974 Mar;129(3):233–239. doi: 10.1093/infdis/129.3.233. [DOI] [PubMed] [Google Scholar]
- McDonald W. I., Halliday A. M. Diagnosis and classification of multiple sclerosis. Br Med Bull. 1977 Jan;33(1):4–9. [PubMed] [Google Scholar]
- Norrby E., Vandvik B. Measles and multiple sclerosis. Proc R Soc Med. 1974 Nov;67(11):1129–1132. doi: 10.1177/003591577406701118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne F. E., Baublis J. V., Itabashi H. H. Isolation of measles virus from cell cultures of brain from a patient with subacute sclerosing panencephalitis. N Engl J Med. 1969 Sep 11;281(11):585–589. doi: 10.1056/NEJM196909112811103. [DOI] [PubMed] [Google Scholar]
- Perrin L. H., Tishon A., Oldstone M. B. Immunologic injury in measles virus infection. III. Presence and characterization of human cytotoxic lymphocytes. J Immunol. 1977 Jan;118(1):282–290. [PubMed] [Google Scholar]
- Tourtellotte W. On cerebrospinal fluid immunoglobulin-G (IgG) quotients in multiple sclerosis and other diseases. A review and a new formula to estimate the amount of IgG synthesized per day by the central nervous system. J Neurol Sci. 1970 Mar;10(3):279–304. doi: 10.1016/0022-510x(70)90156-5. [DOI] [PubMed] [Google Scholar]
- Utermohlen V., Zabriskie J. B. A suppression of cellular immunity in patients with multiple sclerosis. J Exp Med. 1973 Dec 1;138(6):1591–1596. doi: 10.1084/jem.138.6.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdimarsson H., Agnarsdottir G., Lachmann P. J. Cellular immunity in subacute sclerosing panencephalitis. Proc R Soc Med. 1974 Nov;67(11):1125–1129. doi: 10.1177/003591577406701117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandvik B., Norrby E., Nordal H. J., Degré M. Oligoclonal measles virus-specific IgG antibodies isolated from cerebrospinal fluids, brain extracts, and sera from patients with subacute sclerosing panencephalitis and multiple sclerosis. Scand J Immunol. 1976;5(8):979–992. doi: 10.1111/j.1365-3083.1976.tb03050.x. [DOI] [PubMed] [Google Scholar]
- Wainberg M. A., Markson Y., Weiss D. W., Doljanski F. Cellular immunity against Rous sarcomas of chickens. Preferential reactivity against autochthonous target cells as determined by lymphocyte adherence and cytotoxicity tests in vitro. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3565–3569. doi: 10.1073/pnas.71.9.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]



