Abstract
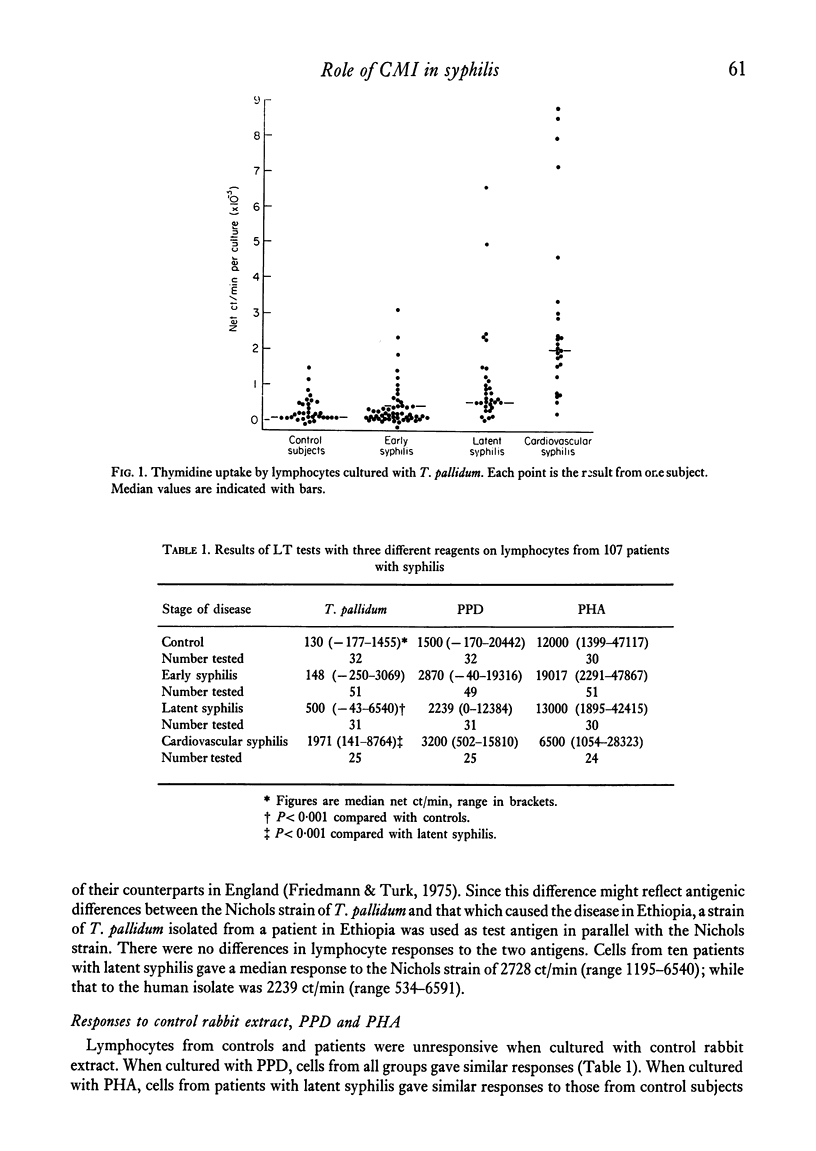
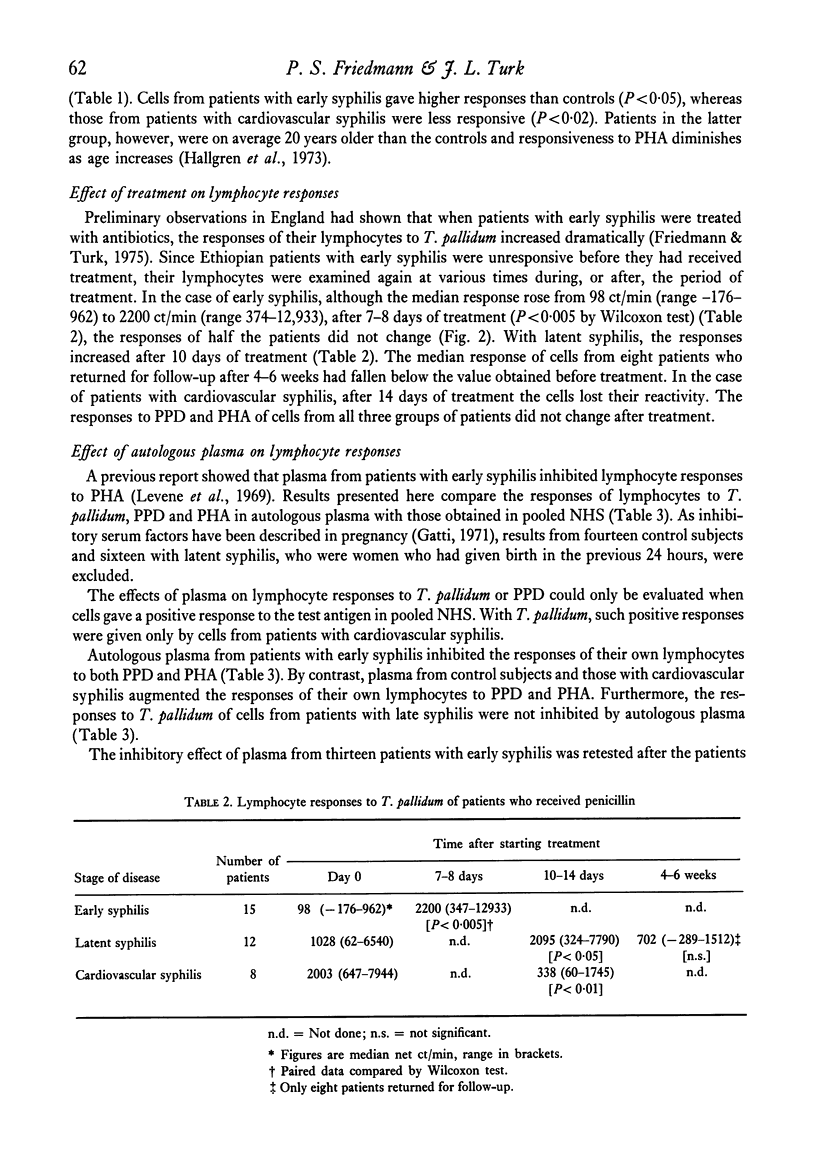
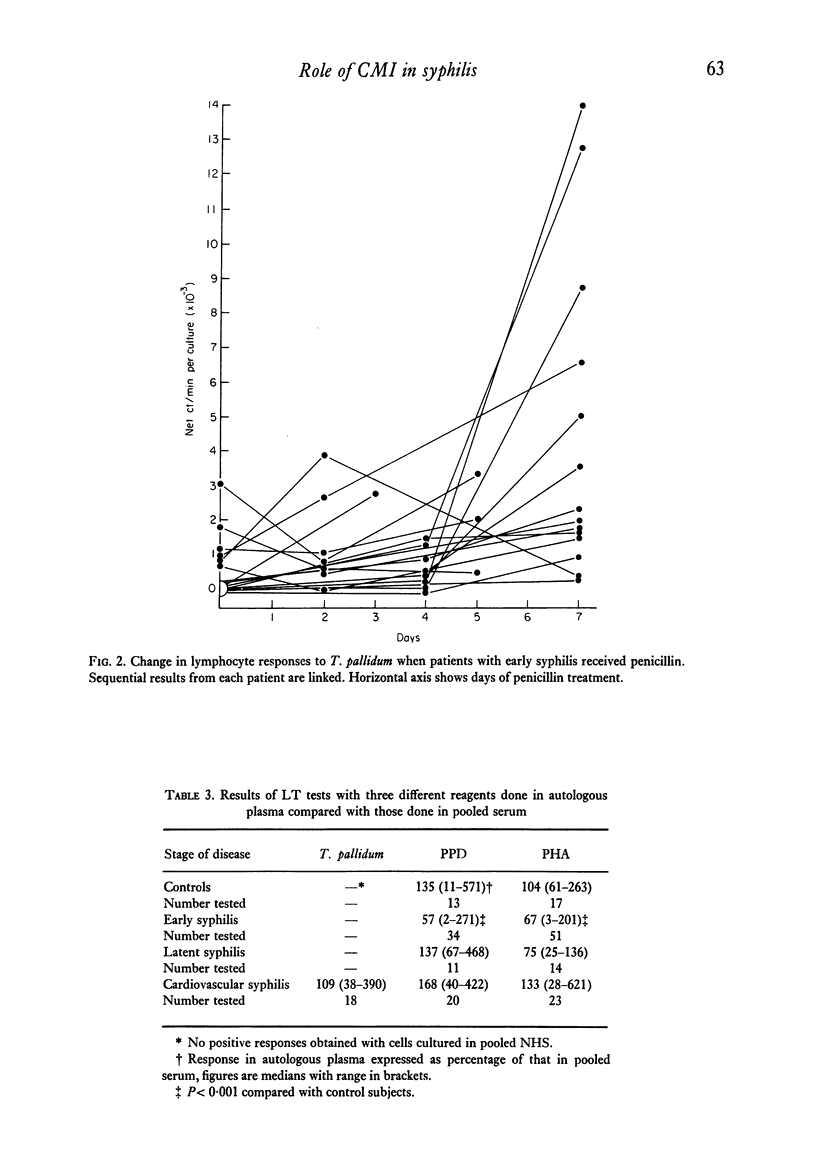
The lymphocyte transformation test was used to assess cell-mediated immune reactivity in 107 Ethiopian patients with syphilis. Lymphocytes from patients with early syphilis were unreactive to the Nichols strain of Treponema pallidum, which was in marked contrast to previous findings in similar patients in England. Lymphocytes obtained from patients with late syphilis, however, were reactive. The responses elicited by a strain of T. pallidum isolated from an Ethiopian with early syphilis did not differ from those with the usual Nichols strain. About half the patients with early syphilis who had received antibiotic treatment for 8 days showed an increase of lymphocyte reactivity towards T. pallidum, although responses to PPD and PHA were unchanged.
Plasma from patients with syphilis was examined for its capacity to inhibit lymphocyte responses in vitro. Although plasma from people with late (cardiovascular) syphilis did not differ from controls, plasma from patients with early syphilis inhibited the responses of their own cells to both PPD and PHA. The inhibitory effect on PHA responses was abrogated after the patients had received antibiotic treatment for 1 week.
The significance of the differences in lymphocyte reactivity observed between Ethiopians with syphilis and their counterparts in England is discussed with regard to a possible explanation of the differences in the natural history of the disease in the two countries.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop N. H., Miller J. N. Humoral immunity in experimental syphilis. I. The demonstration of resistance conferred by passive immunization. J Immunol. 1976 Jul;117(1):191–196. [PubMed] [Google Scholar]
- Bjune G., Barnetson R. S., Ridley D. S., Kronvall G. Lymphocyte transformation test in leprosy; correlation of the response with inflammation of lesions. Clin Exp Immunol. 1976 Jul;25(1):85–94. [PMC free article] [PubMed] [Google Scholar]
- Bryceson A. D., Bray R. S., Wolstencroft R. A., Dumonde D. C. Immunity in cutaneous leishmaniasis of the guinea-pig. Clin Exp Immunol. 1970 Sep;7(3):301–341. [PMC free article] [PubMed] [Google Scholar]
- FERREIRA-MARQUES J. CONTRIBUTION A L'ETUDE DES MALADIES VENERIENNES ET CUTANEES A ADDIS-ABABA. Dermatol Trop Ecol Geogr. 1964 Jul-Sep;30:139–151. [PubMed] [Google Scholar]
- Fleer A., van der Hart M., Blok-Schut B. J., Schellekens P. T. Correlation of PPD and BCG-induced leukocyte migration inhibition, delayed cutaneous hypersensitivity, lymphocyte transformation in vitro and humoral antibodies to PPD in man. Eur J Immunol. 1976 Mar;6(3):163–167. doi: 10.1002/eji.1830060305. [DOI] [PubMed] [Google Scholar]
- Friedman P. S., Wright D. J. Observations on syphilis in Addis Ababa. 2. Prevalence and natural history. Br J Vener Dis. 1977 Oct;53(5):276–280. doi: 10.1136/sti.53.5.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- From E., Thestrup-Pedersen K., Thulin H. Reactivity of lymphocytes from patients with syphilis towards T. pallidum antigen in the leucocyte migration and lymphocyte transformation tests. Br J Vener Dis. 1976 Aug;52(4):224–229. doi: 10.1136/sti.52.4.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulford K. W., Brostoff J. Leucocyte migration and cell-mediated immunity in syphilis. Br J Vener Dis. 1972 Dec;48(6):483–488. doi: 10.1136/sti.48.6.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti R. A. Serum inhibitors of lymphocyte responses. Lancet. 1971 Jun 26;1(7713):1351–1352. doi: 10.1016/s0140-6736(71)91906-4. [DOI] [PubMed] [Google Scholar]
- Hallgren H. M., Buckley C. E., 3rd, Gilbertsen V. A., Yunis E. J. Lymphocyte phytohemagglutinin responsiveness, immunoglobulins and autoantibodies in aging humans. J Immunol. 1973 Oct;111(4):1101–1107. [PubMed] [Google Scholar]
- Levene G. M., Turk J. L., Wright D. J., Grimble A. G. Reduced lymphocyte transformation due to a plasma factor in patients with active syphilis. Lancet. 1969 Aug 2;2(7614):246–247. doi: 10.1016/s0140-6736(69)90010-5. [DOI] [PubMed] [Google Scholar]
- Musher D. M., Schell R. F., Jones R. H., Jones A. M. Lymphocyte transformation in syphilis: an in vitro correlate of immune suppression in vivo? Infect Immun. 1975 Jun;11(6):1261–1264. doi: 10.1128/iai.11.6.1261-1264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees R. J., Waters M. F., Weddell A. G., Palmer E. Experimental lepromatous leprosy. Nature. 1967 Aug 5;215(5101):599–602. doi: 10.1038/215599a0. [DOI] [PubMed] [Google Scholar]
- Sykes J. A., Miller J. N. Intracellular location of Treponema pallidum (Nichols strain) in the rabbit testis. Infect Immun. 1971 Sep;4(3):307–314. doi: 10.1128/iai.4.3.307-314.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser R. S., Erickson D., Perine P. L., Pearsall N. N. Immunity to syphilis: passive transfer in rabbits using serial doses of immune serum. Infect Immun. 1976 May;13(5):1402–1407. doi: 10.1128/iai.13.5.1402-1407.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


