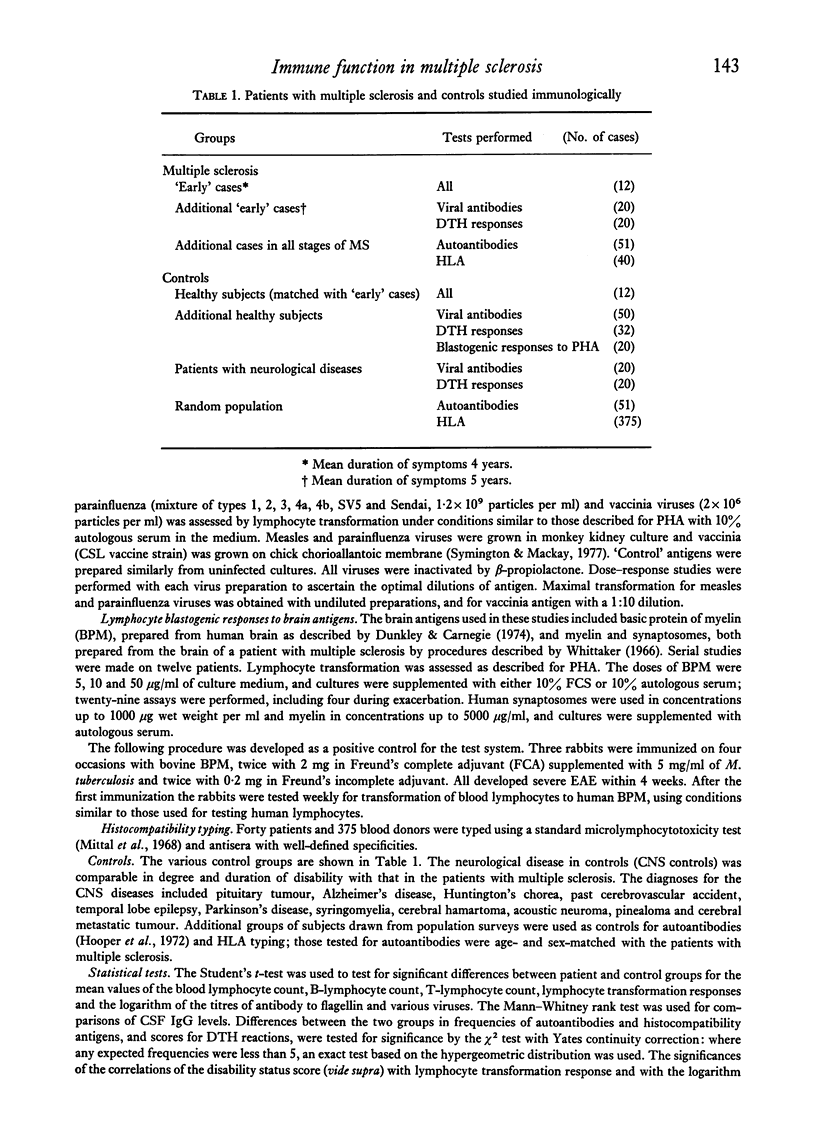
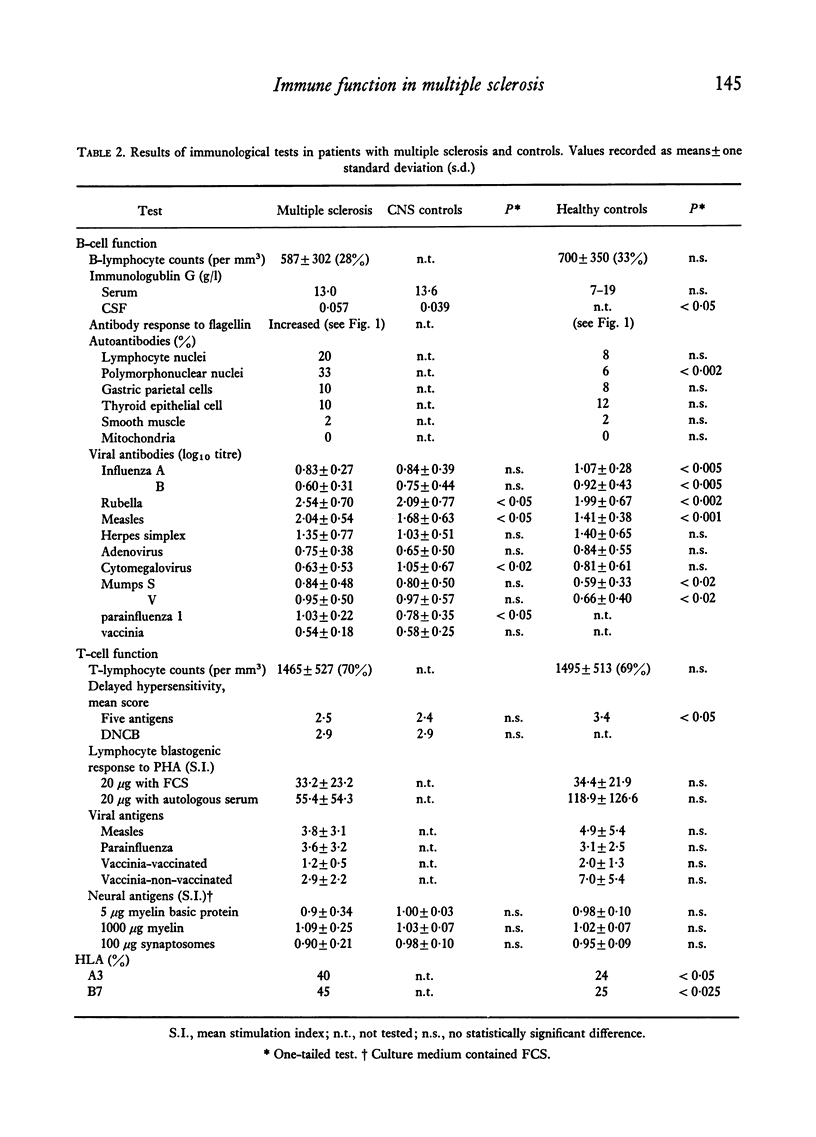
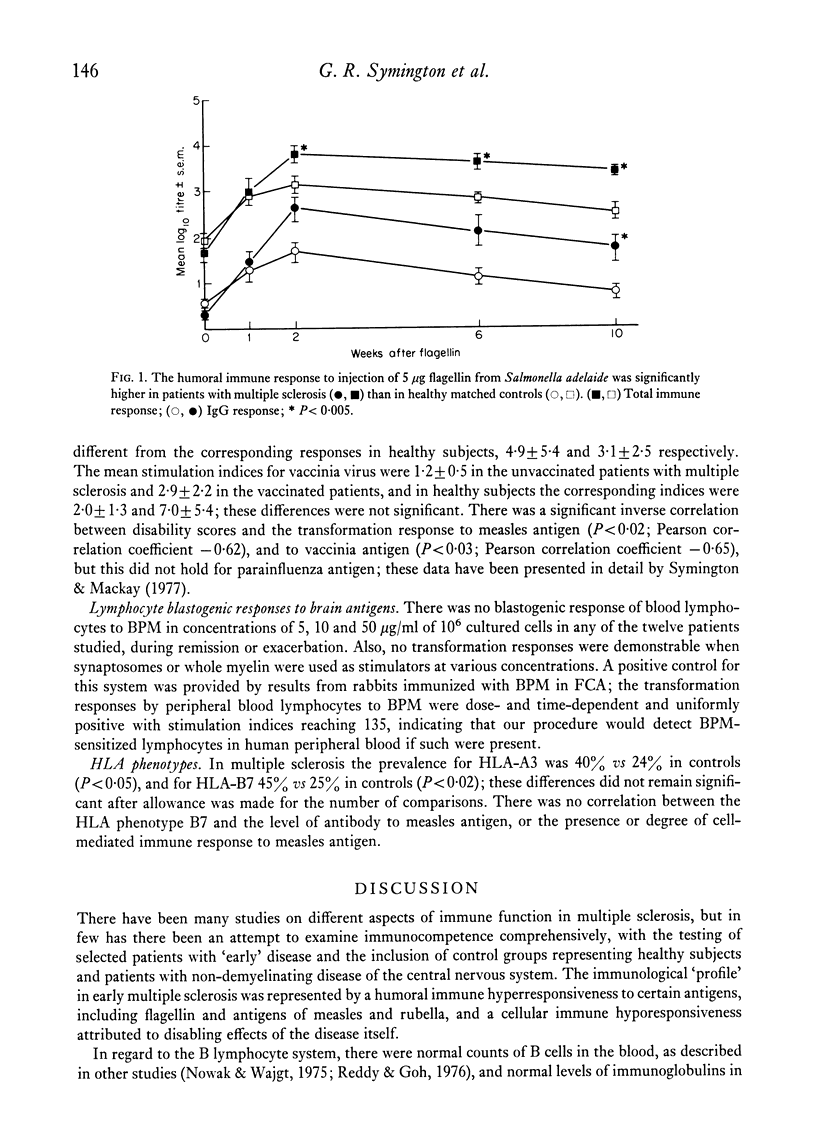
Abstract
An 'immunological profile' of various indices of B-cell function and T-cell function was developed for the 'early' case of multiple sclerosis (MS). This was compared against two groups of controls comprising age and sex-matched healthy subjects, and patients with other disabling neurological diseases (CNS controls) who were matched for age, sex, and type and duration of disability. Some indices of humoral immune responsiveness, such as the induced primary response to monomeric flagellin and the 'resting' levels of antibody to measles and rubella viruses, showed significant augmentation. Cellular immune deficits were attributed to an illness effect per se because (a) cell-mediated immunity was depressed, but only when compared with that of healthy subjects and not when compared with that of the CNS controls, and (b) transformation responses of lymphocytes to viral antigens were inversely related to disability status. The abnormalities in humoral immune responses demonstrable in this study do not provide an explanation for this disease; if there is a relevant 'immunological fault', the nature of this needs to be sought from within the neuraxis rather than from the systemic circulation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADA G. L., NOSSAL G. J., PYE J., ABBOT A. ANTIGENS IN IMMUNITY. I. PREPARATION AND PROPERTIES OF FLAGELLAR ANTIGENS FROM SALMONELLA ADELAIDE. Aust J Exp Biol Med Sci. 1964 Jun;42:267–282. [PubMed] [Google Scholar]
- ADAMS J. M., IMAGAWA D. T. Measles antibodies in multiple sclerosis. Proc Soc Exp Biol Med. 1962 Dec;111:562–566. doi: 10.3181/00379727-111-27855. [DOI] [PubMed] [Google Scholar]
- Allen J. C., Sheremata W., Cosgrove J. B., Osterland K., Shea M. Cerebrospinal fluid T and B lymphocyte kinetics related to exacerbations of multiple sclerosis. Neurology. 1976 Jun;26(6 Pt 1):579–583. doi: 10.1212/wnl.26.6.579. [DOI] [PubMed] [Google Scholar]
- Bernard C. C., Leydon J., Mackay I. R. T cell necessity in the pathogenesis of experimental autoimmune encephalomyelitis in mice. Eur J Immunol. 1976 Sep;6(9):655–660. doi: 10.1002/eji.1830060912. [DOI] [PubMed] [Google Scholar]
- Brody J. A., Sever J. L., Edgar A., McNew J. Measles antibody titers of multiple sclerosis patients and their siblings. Neurology. 1972 May;22(5):492–499. doi: 10.1212/wnl.22.5.492. [DOI] [PubMed] [Google Scholar]
- Brostoff J. A simple technique for counting rosettes using acridine orange. J Immunol Methods. 1974 Aug;5(3):303–303. doi: 10.1016/0022-1759(74)90115-x. [DOI] [PubMed] [Google Scholar]
- Carp R. I., Licursi P. C., Merz P. A., Merz G. S. Decreased percentage of polymorphonuclear neutrophils in mouse peripheral blood after inoculation with material from multiple sclerosis patients. J Exp Med. 1972 Sep 1;136(3):618–629. doi: 10.1084/jem.136.3.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciongoli A. K., Platz P., Dupont B., Svejgaard A., Fog T., Jersild C. Letter: Lack of antigen response to myxoviruses in multiple sclerosis. Lancet. 1973 Nov 17;2(7838):1147–1147. [PubMed] [Google Scholar]
- Colby S. P., Sheremata W., Bain B., Eylar E. H. Cellular hypersensitivity in attacks of multiple sclerosis. I. A comparative study of migration inhibitory factor production and lymphoblastic transformation in response to myelin basic protein in multiple sclerosis. Neurology. 1977 Feb;27(2):132–139. doi: 10.1212/wnl.27.2.132. [DOI] [PubMed] [Google Scholar]
- Compston D. A., Batchelor J. R., McDonald W. I. B-lymphocyte alloantigens associated with multiple sclerosis. Lancet. 1976 Dec 11;2(7998):1261–1265. doi: 10.1016/s0140-6736(76)92027-4. [DOI] [PubMed] [Google Scholar]
- Davis L. E., Hersh E. M., Curtis J. E., Lynch R. E., Ziegler D. K., Neumann J. W., Chin T. D. Immune status of patients with multiple sclerosis. Analysis of primary and established immune responses in 24 patients. Neurology. 1972 Oct;22(10):989–997. doi: 10.1212/wnl.22.9.989. [DOI] [PubMed] [Google Scholar]
- Dean G. Annual incidence, prevalence, and mortality of multiple sclerosis in white South-African-born and in white immigrants to South Africa. Br Med J. 1967 Jun 17;2(5554):724–730. doi: 10.1136/bmj.2.5554.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHEY J. L., MCKELVEY E. M. QUANTITATIVE DETERMINATION OF SERUM IMMUNOGLOBULINS IN ANTIBODY-AGAR PLATES. J Immunol. 1965 Jan;94:84–90. [PubMed] [Google Scholar]
- FIELD E. J., GREEN C. A., MILLER H. Response of normal and multiple sclerotic subjects to typhoid-paratyphoid vaccine injections. J Neurol Neurosurg Psychiatry. 1961 Feb;24:78–79. doi: 10.1136/jnnp.24.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper B., Whittingham S., Mathews J. D., Mackay I. R., Curnow D. H. Autoimmunity in a rural community. Clin Exp Immunol. 1972 Sep;12(1):79–87. [PMC free article] [PubMed] [Google Scholar]
- JOHNSON R. T., MILLER H. Dermal sensitivity tests in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1963 Apr;26:151–153. doi: 10.1136/jnnp.26.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jersild C., Dupont B., Fog T., Platz P. J., Svejgaard A. Histocompatibility determinants in multiple sclerosis. Transplant Rev. 1975;22:148–163. doi: 10.1111/j.1600-065x.1975.tb01556.x. [DOI] [PubMed] [Google Scholar]
- Kaplan M. E., Clark C. An improved rosetting assay for detection of human T lymphocytes. J Immunol Methods. 1974 Jul;5(2):131–135. doi: 10.1016/0022-1759(74)90003-9. [DOI] [PubMed] [Google Scholar]
- Koldovsky U., Koldovsky P., Henle G., Henle W., Ackermann R., Haase G. Multiple sclerosis-associated agent: transmission to animals and some properties of the agent. Infect Immun. 1975 Dec;12(6):1355–1366. doi: 10.1128/iai.12.6.1355-1366.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Källén B., Nilsson O., Thelin C. Effect of encephalitogenic protein on migration in agarose of leukocytes from patients with multiple sclerosis. Variable effect of the antigen in a large dose range, with a literature review. Acta Neurol Scand. 1977 Jan;55(1):33–46. doi: 10.1111/j.1600-0404.1977.tb05625.x. [DOI] [PubMed] [Google Scholar]
- Lamoureux G., Giard N., Jolicoeur R., Toughlian V., Desrosiers M. Immunological features in multiple sclerosis. Br Med J. 1976 Jan 24;1(6003):183–186. doi: 10.1136/bmj.1.6003.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Lee A. K., Mackay I. R., Rowley M. J., Yap C. Y. Measurement of antibody-producing capacity to flagellin in man. IV. Studies in autoimmune disease, allergy and after azathioprine treatment. Clin Exp Immunol. 1971 Oct;9(4):507–518. [PMC free article] [PubMed] [Google Scholar]
- Leibowitz U., Kahana E., Alter M. Multiple sclerosis in immigrant and native populations of Israel. Lancet. 1969 Dec 20;2(7634):1323–1325. doi: 10.1016/s0140-6736(69)90863-0. [DOI] [PubMed] [Google Scholar]
- Levinson A. I., Lisak R. P., Zweiman B. Immunologic characterization of cerebrospinal fluid lymphocytes: preliminary report. Neurology. 1976 Jul;26(7):693–695. doi: 10.1212/wnl.26.7.693. [DOI] [PubMed] [Google Scholar]
- Lisak R. P., Levinson A. I., Zweiman B., Abdou N. I. T and B lymphocytes in multiple sclerosis. Clin Exp Immunol. 1975 Oct;22(1):30–34. [PMC free article] [PubMed] [Google Scholar]
- Lisak R. P. Multiple sclerosis: immunologic aspects. Ann Clin Lab Sci. 1975 Sep-Oct;5(5):324–329. [PubMed] [Google Scholar]
- Mackay I. R., Carnegie P. R., Coates A. S. Immunopathological comparisons between experimental autoimmune encephalomyelitis and multiple sclerosis. Clin Exp Immunol. 1973 Dec;15(4):471–482. [PMC free article] [PubMed] [Google Scholar]
- Mittal K. K., Mickey M. R., Singal D. P., Terasaki P. I. Serotyping for homotransplantation. 18. Refinement of microdroplet lymphocyte cytotoxicity test. Transplantation. 1968 Nov;6(8):913–927. doi: 10.1097/00007890-196811000-00006. [DOI] [PubMed] [Google Scholar]
- Nowak J., Wajgt A. Surface markers on lymphocytes of multiple sclerosis patients. Clin Exp Immunol. 1975 Aug;21(2):278–283. [PMC free article] [PubMed] [Google Scholar]
- Oger J. F., Arnason B. G., Wray S. H., Kistler J. P. A study of B and T cells in multiple sclerosis. Neurology. 1975 May;25(5):444–447. doi: 10.1212/wnl.25.5.444. [DOI] [PubMed] [Google Scholar]
- Opelz G., Terasaki P., Myers L., Ellison G., Ebers G., Zabriskie J., Weiner H., Kempe H., Sibley W. The association of HLA antigens A3, B7, and DW2 with 330 multiple sclerosis patients in the United States. Tissue Antigens. 1977 Jan;9(1):54–58. doi: 10.1111/j.1399-0039.1977.tb01080.x. [DOI] [PubMed] [Google Scholar]
- Reddy M. M., Goh K. O. B and T lymphocytes in man. III. B, t, and "null" lymphocytes in multiple sclerosis. Neurology. 1976 Oct;26(10):997–999. doi: 10.1212/wnl.26.10.997. [DOI] [PubMed] [Google Scholar]
- Rowley M. J., Mackay I. R. Measurement of antibody-producing capacity in man. I. The normal response to flagellin from Salmonella adelaide. Clin Exp Immunol. 1969 Oct;5(4):407–418. [PMC free article] [PubMed] [Google Scholar]
- Sever J. L., Kurtzke J. F. Delayed dermal hypersensitivity to measles and mumps antigens among multiple sclerosis and control patients. Neurology. 1969 Feb;19(2):113–115. doi: 10.1212/wnl.19.2.113. [DOI] [PubMed] [Google Scholar]
- Thompson C. D., Whittingham S., Mackay I. R., Khoo S. K., Toh B. H., Stagg R. J. Quantitation of cell-mediated immunity: responses to dinitrochlorobenzene and ubiquitous antigens. Can Med Assoc J. 1975 May 3;112(9):1078–1081. [PMC free article] [PubMed] [Google Scholar]
- Toh B. H., Roberts-Thomson I. C., Mathews J. D., Whittingham S., Mackay I. R. Depression of cell-mediated immunity in old age and the immunopathic diseases, lupus erythematosus, chronic hepatitis and rheumatoid arthritis. Clin Exp Immunol. 1973 Jun;14(2):193–202. [PMC free article] [PubMed] [Google Scholar]
- Utermohlen V., Winfield J. B., Zabriskie J. B., Kunkel H. G. A depression of cell-mediated immunity to measles antigen in patients with systemic lupus erythematosus. J Exp Med. 1974 Apr 1;139(4):1019–1024. doi: 10.1084/jem.139.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandvik B., Natvig J. B., Wiger D. IgG1 subclass restriction of oligoclonal IgG from cerebrospinal fluids and brain extracts in patients with multiple sclerosis and subacute encephalitides. Scand J Immunol. 1976;5(4):427–436. doi: 10.1111/j.1365-3083.1976.tb00297.x. [DOI] [PubMed] [Google Scholar]
- Whittaker V. P. Some properties of synaptic membranes isolated from the central nervous system. Ann N Y Acad Sci. 1966 Jul 14;137(2):982–998. doi: 10.1111/j.1749-6632.1966.tb50211.x. [DOI] [PubMed] [Google Scholar]
- Whittingam S., Mackay I. R. Laboratory methods for diagnosis of autoimmune disease. Med J Aust. 1969 Jun 7;1(23):1200–1205. doi: 10.5694/j.1326-5377.1969.tb62269.x. [DOI] [PubMed] [Google Scholar]


