Abstract
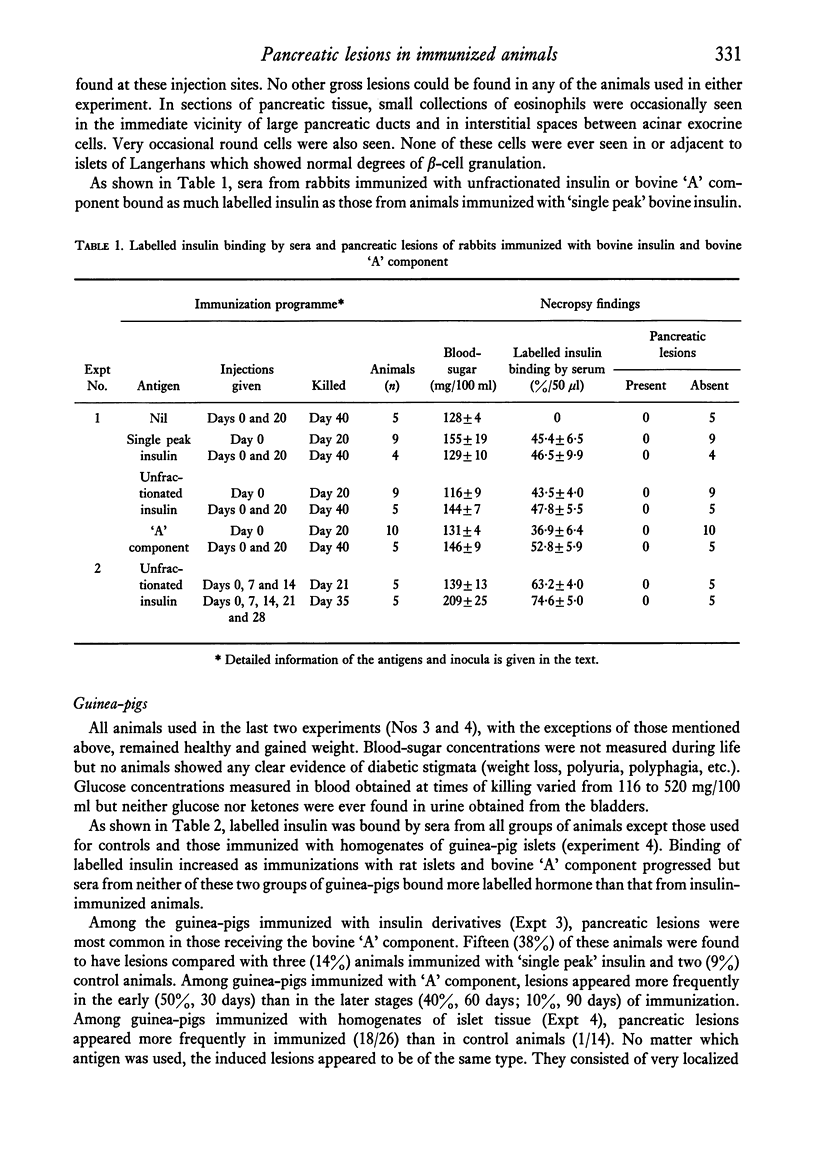
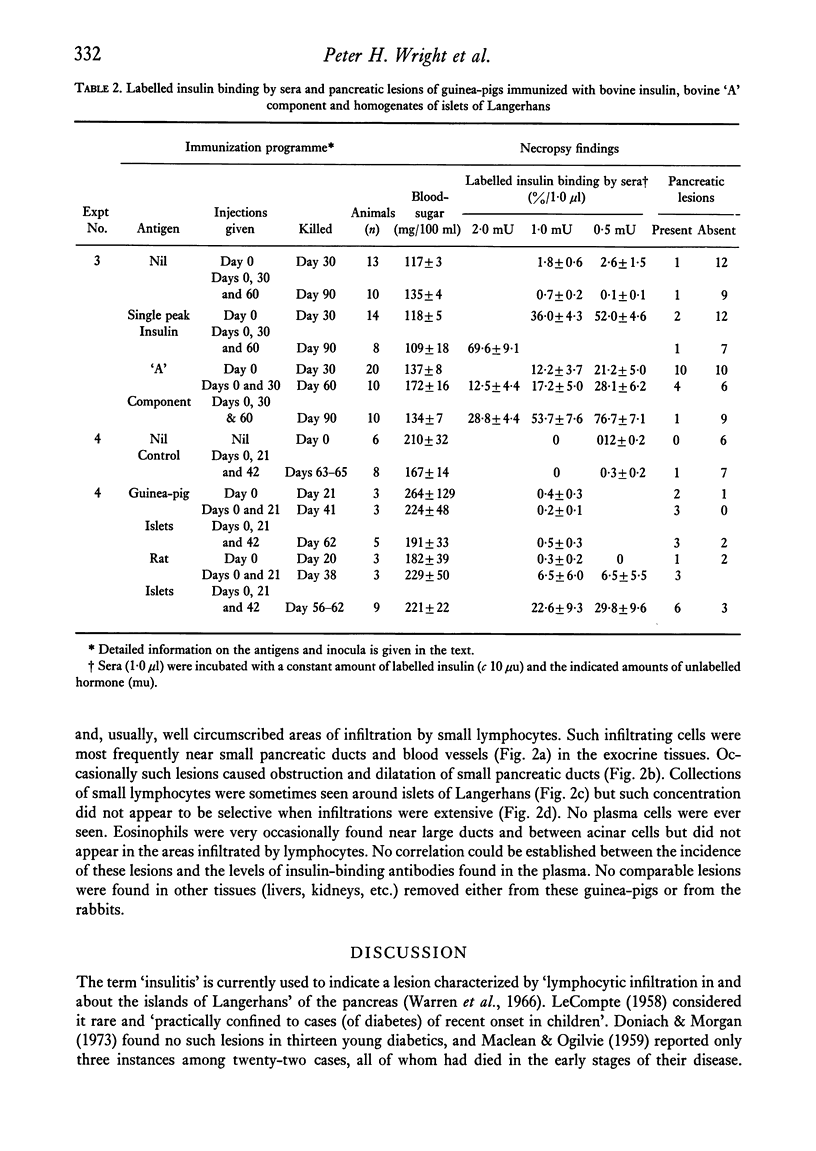
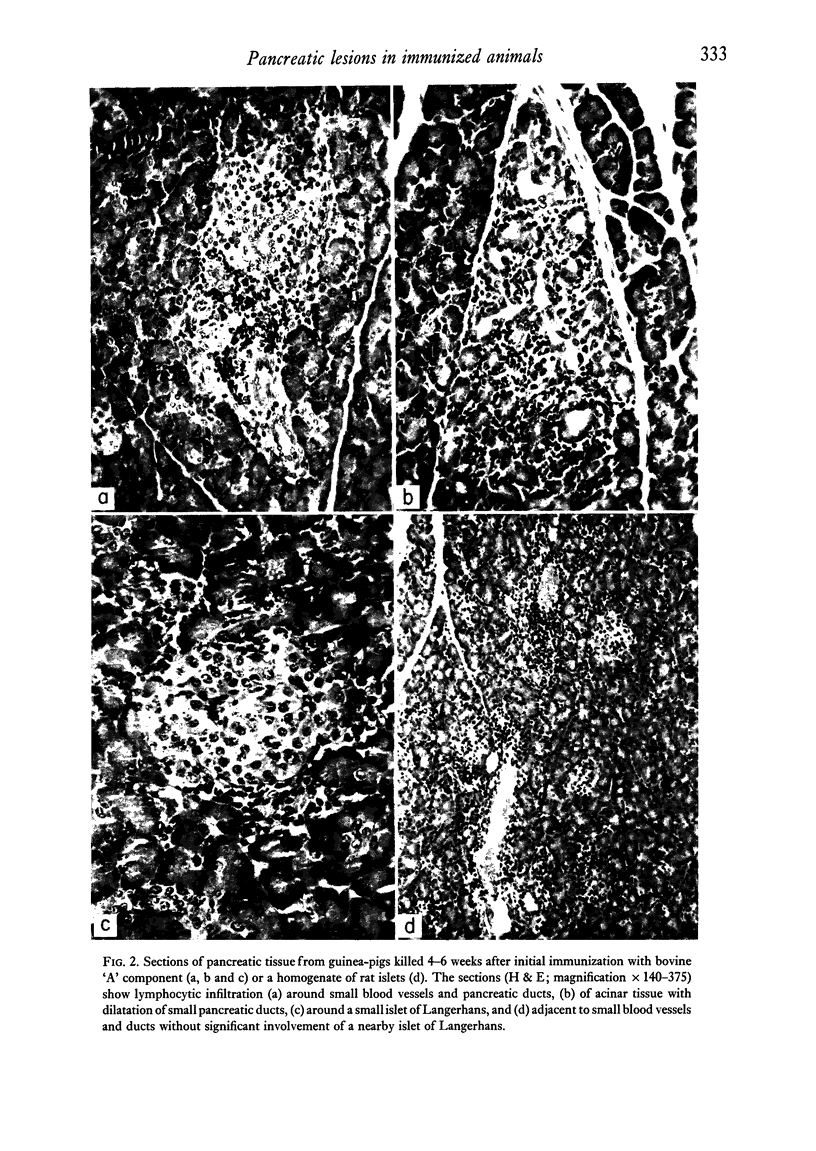
Rabbits and guinea-pigs were immunized with various pancreatic antigens in Freund's adjuvant. Rabbits received unfractionated bovine insulin and the "A" component and "single peak" insulin separated from it by gel-filtration. All produced antibodies capable of reacting with porcine insulin but none were found to have pancreatic lesions when killed up to 6 weeks after initial injection. Guinea-pigs immunized with bovine "A" component developed pancreatic peri-ductulitis which appeared most frequently (10/20) in animals killed 30 days after a single injection and less frequently in animals killed after 60 (4/10) and 90(1/10) days. Similar lesions were found in only a small proportion of control animals (2/23) or of guinea-pigs immunized with single peak bovine insulin (3/22). Guinea-pigs immunized with homogenates of homologous and heterologous islets of Langerhans developed signs of peri-ductulitis in a high proportion of animals killed up to about 60 days after first injection (18/26). None of these animals exhibited clearly defined signs of diabetes mellitus and the incidence of induced lesions could not be correlated with levels of circulating insulin-binding antibodies.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN E. E. Infectious origin of juvenile diabetes. Arch Pediatr. 1956 Jun;73(6):191–198. [PubMed] [Google Scholar]
- Burch G. E., Tsui C. Y., Harb J. M., Colcolough H. L. Pathologic findings in the pancreas of mice infected with coxsackievirus B4. Arch Intern Med. 1971 Jul;128(1):40–47. [PubMed] [Google Scholar]
- Coleman T. J., Gamble D. R., Taylor K. W. Diabetes in mice after Coxsackie B 4 virus infection. Br Med J. 1973 Jul 7;3(5870):25–27. doi: 10.1136/bmj.3.5870.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craighead J. E., Higgins D. A. Genetic influences affecting the occurrence of a diabetes mellitus-like disease in mice infected with the encephalomyocarditis virus. J Exp Med. 1974 Feb 1;139(2):414–426. doi: 10.1084/jem.139.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craighead J. E., Steinke J. Diabetes mellitus-like syndrome in mice infected with encephalomyocarditis virus. Am J Pathol. 1971 Apr;63(1):119–134. [PMC free article] [PubMed] [Google Scholar]
- Doniach I., Morgan A. G. Islets of Langerhans in juvenile diabetes mellitus. Clin Endocrinol (Oxf) 1973 Jul;2(3):233–248. doi: 10.1111/j.1365-2265.1973.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Freytag G., Jansen F. K., Klöppel G. Immune reactions to fractions of crystalline insulin. I. Significance of lymphocytic infiltrates in the endocrine and exocrine pancreas of mice. Diabetologia. 1973 Jun;9(3):185–190. doi: 10.1007/BF01219781. [DOI] [PubMed] [Google Scholar]
- Gamble D. R., Kinsley M. L., FitzGerald M. G., Bolton R., Taylor K. W. Viral antibodies in diabetes mellitus. Br Med J. 1969 Sep 13;3(5671):627–630. doi: 10.1136/bmj.3.5671.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble D. R., Taylor K. W. Seasonal incidence of diabetes mellitus. Br Med J. 1969 Sep 13;3(5671):631–633. doi: 10.1136/bmj.3.5671.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965 Oct;14(10):619–633. doi: 10.2337/diab.14.10.619. [DOI] [PubMed] [Google Scholar]
- Goldstein D. E., Drash A., Gibbs J., Blizzard R. M. Diabetes mellitus: the incidence of circulating antibodies against thyroid, gastric, and adrenal tissue. J Pediatr. 1970 Aug;77(2):304–306. doi: 10.1016/s0022-3476(70)80342-0. [DOI] [PubMed] [Google Scholar]
- Heydinger D. K., Lacy P. E. Islet cell changes in the rat following injection of homogenized islets. Diabetes. 1974 Jul;23(7):579–582. doi: 10.2337/diab.23.7.579. [DOI] [PubMed] [Google Scholar]
- Irvine W. J., Clarke B. F., Scarth L., Cullen D. R., Duncan L. J. Thyroid and gastric autoimmunity in patients with diabetes mellitus. Lancet. 1970 Jul 25;2(7665):163–168. doi: 10.1016/s0140-6736(70)92531-6. [DOI] [PubMed] [Google Scholar]
- Klöppel G., Altenähr E., Freytag G., Jansen F. K. Immune insulitis and manifest diabetes mellitus. Studies on the course of immune insulitis and the induction of diabetes mellitus in rabbits immunized with insulin. Virchows Arch A Pathol Anat Histol. 1974;364(4):333–346. doi: 10.1007/BF00432731. [DOI] [PubMed] [Google Scholar]
- Klöppel G., Altenähr E., Freytag G. Studies on ultrastructure and immunology of the insulitis in rabbits immunized with insulin. Virchows Arch A Pathol Pathol Anat. 1972;356(1):1–15. doi: 10.1007/BF00543553. [DOI] [PubMed] [Google Scholar]
- LECOMPTE P. M. Insulitis in early juvenile diabetes. AMA Arch Pathol. 1958 Oct;66(4):450–457. [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- LeCompte P. M., Steinke J., Soeldner J. S., Renold A. E. Changes in the islets of Langerhans in cows injected with heterologous and homologous insulin. Diabetes. 1966 Aug;15(8):586–596. doi: 10.2337/diab.15.8.586. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Grodsky G. M., Caplan J., Craw L. Experimental immune diabetes in the rabbit. Light, fluorescence, and electron microscopic studies. Am J Pathol. 1969 Dec;57(3):597–616. [PMC free article] [PubMed] [Google Scholar]
- Lendrum R., Walker G., Gamble D. R. Islet-cell antibodies in juvenile diabetes mellitus of recent onset. Lancet. 1975 Apr 19;1(7912):880–882. doi: 10.1016/s0140-6736(75)91683-9. [DOI] [PubMed] [Google Scholar]
- Lindall A., Steffes M., Sorenson R. Immunoassayable insulin content of subcellular fractions of rat islets. Endocrinology. 1969 Aug;85(2):218–223. doi: 10.1210/endo-85-2-218. [DOI] [PubMed] [Google Scholar]
- MACLEAN N., OGILVIE R. F. Observations on the pancreatic islet tissue of young diabetic subjects. Diabetes. 1959 Mar-Apr;8(2):83–91. doi: 10.2337/diab.8.2.83. [DOI] [PubMed] [Google Scholar]
- Makulu D. R., Wright P. Immune response to insulin in guinea pigs. Metabolism. 1971 Aug;20(8):770–781. doi: 10.1016/s0026-0495(71)80007-0. [DOI] [PubMed] [Google Scholar]
- Nerup J., Andersen O. O., Bendixen G., Egeberg J., Gunnarsson R., Kromann H., Poulsen J. E. Glucose intolerance and islet damage in mice immunized with homologous endocrine pancreas--a preliminary communication. Horm Metab Res. 1974 May;6(3):173–175. doi: 10.1055/s-0028-1093865. [DOI] [PubMed] [Google Scholar]
- Nerup J., Andersen O. O., Bendixen G., Egeberg J., Poulsen J. E. Anti-pancreatic, cellular hypersensitivity in diabetes mellitus. Antigenic activity of fetal calf pancreas and correlation with clinical type of diabetes. Acta Allergol. 1973 Oct;28(4):223–230. doi: 10.1111/j.1398-9995.1973.tb01443.x. [DOI] [PubMed] [Google Scholar]
- Nerup J., Andersen O. O., Bendixen G., Egeberg J., Poulsen J. E., Vilien M., Westrup M. Anti-pancreatic, cellular hypersensitivity in diabetes mellitus. Experimental induction of anti-pancreatic, cellular hypersensitivity and associated morphological B-cell changes in the rat. Acta Allergol. 1973 Oct;28(4):231–249. doi: 10.1111/j.1398-9995.1973.tb01444.x. [DOI] [PubMed] [Google Scholar]
- Nerup J., Binder C. Thyroid, gastric and adrenal auto-immunity in diabetes mellitus. Acta Endocrinol (Copenh) 1973 Feb;72(2):279–286. doi: 10.1530/acta.0.0720279. [DOI] [PubMed] [Google Scholar]
- Nerup J., Platz P., Andersen O. O., Christy M., Lyngsoe J., Poulsen J. E., Ryder L. P., Nielsen L. S., Thomsen M., Svejgaard A. HL-A antigens and diabetes mellitus. Lancet. 1974 Oct 12;2(7885):864–866. doi: 10.1016/s0140-6736(74)91201-x. [DOI] [PubMed] [Google Scholar]
- Renold A. E., Steinke J., Soeldner J. S., Antoniades H. N., Smith R. E. Immunological response to the prolonged administration of heterologous and homologous insulin in cattle. J Clin Invest. 1966 May;45(5):702–713. doi: 10.1172/JCI105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichtkrull J., Brange J., Christiansen A. H., Hallund O., Heding L. G., Jorgensen K. H. Clinical aspects of insulin--antigenicity. Diabetes. 1972;21(2 Suppl):649–656. doi: 10.2337/diab.21.2.s649. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Oyer P. E. The biosynthesis of insulin and a probable precursor of insulin by a human islet cell adenoma. Proc Natl Acad Sci U S A. 1967 Feb;57(2):473–480. doi: 10.1073/pnas.57.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toreson W. E., Lee J. C., Grodsky G. M. The histopathology of immune diabetes in the rabbit. Am J Pathol. 1968 May;52(5):1099–1115. [PMC free article] [PubMed] [Google Scholar]
- Ungar B., Stocks A. E., Martin F. I., Whittingham S., Mackay I. R. Intrinsic-factor antibody, parietal-cell antibody, and latent pernicious anaemia in diabetes mellitus. Lancet. 1968 Aug 24;2(7565):415–417. doi: 10.1016/s0140-6736(68)90462-5. [DOI] [PubMed] [Google Scholar]



