Abstract
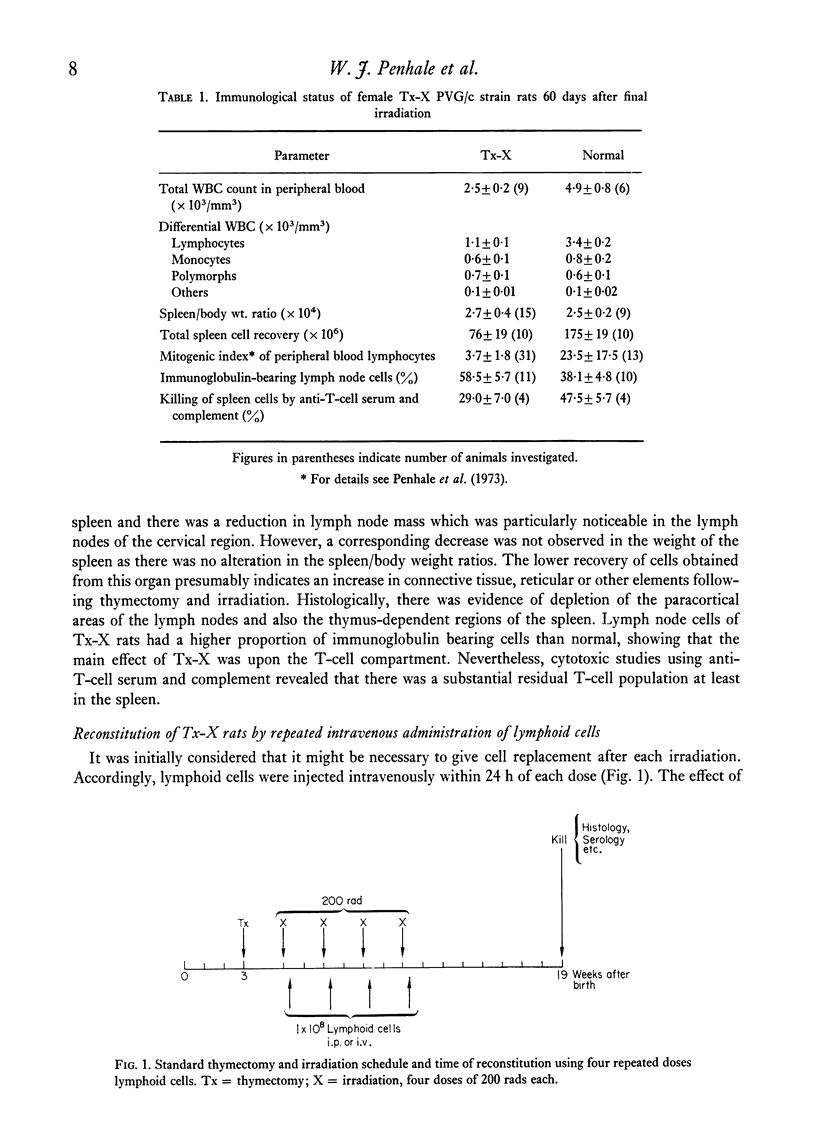
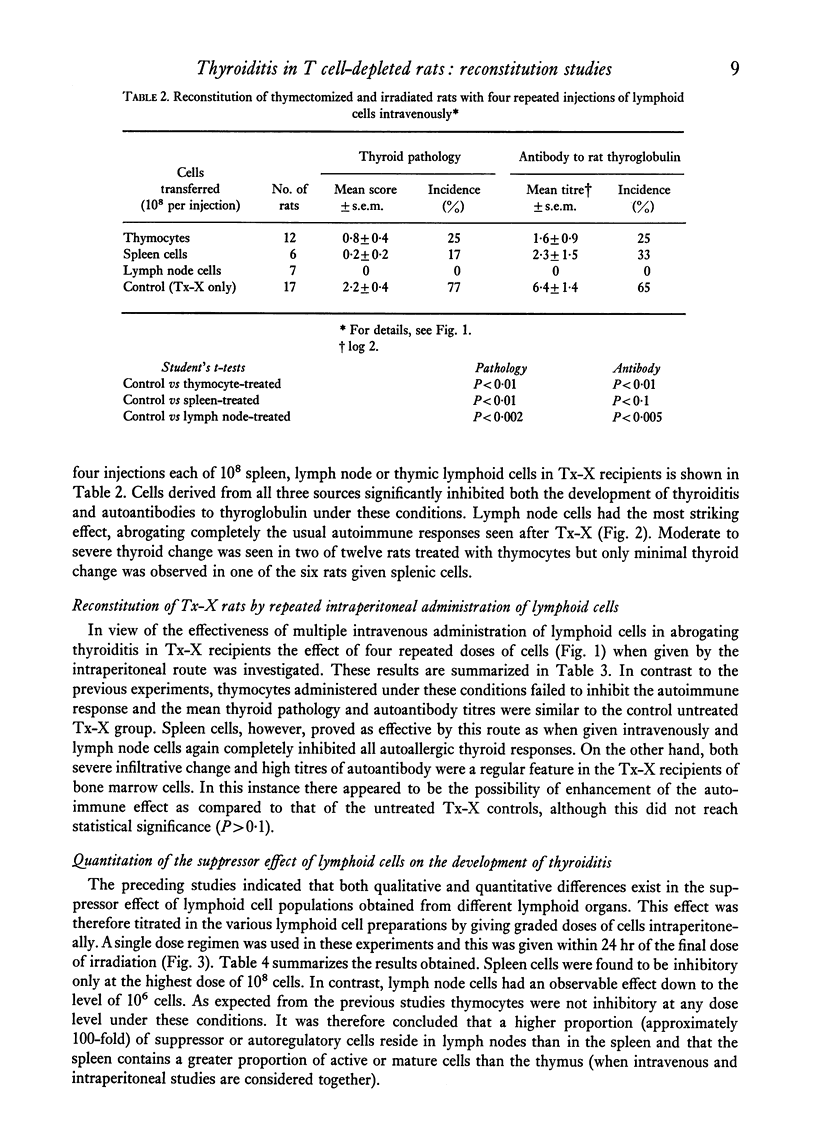
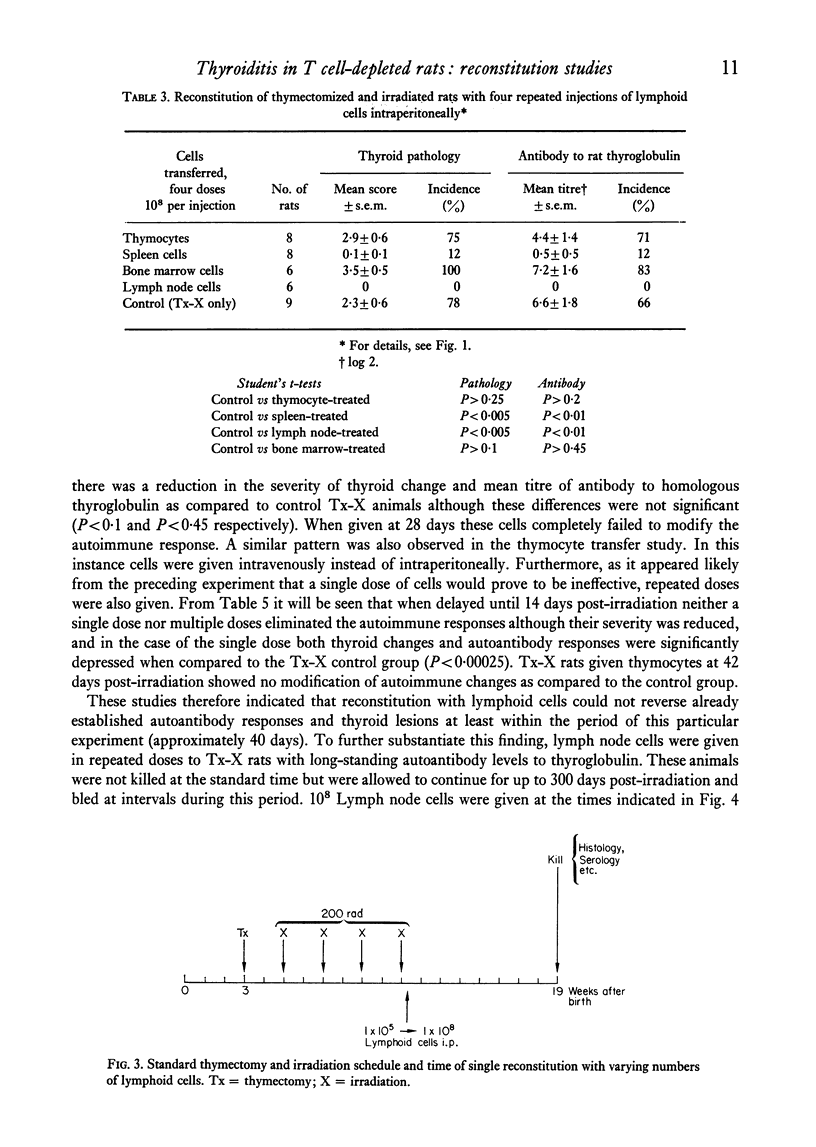
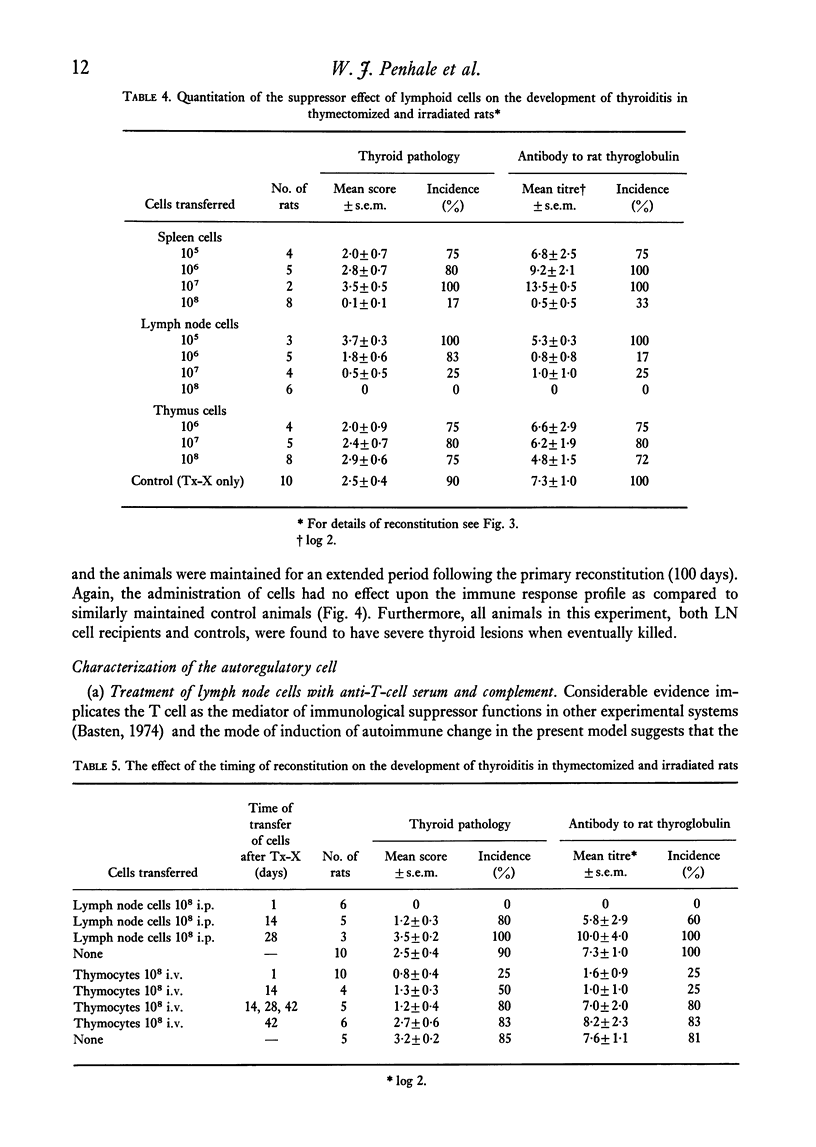
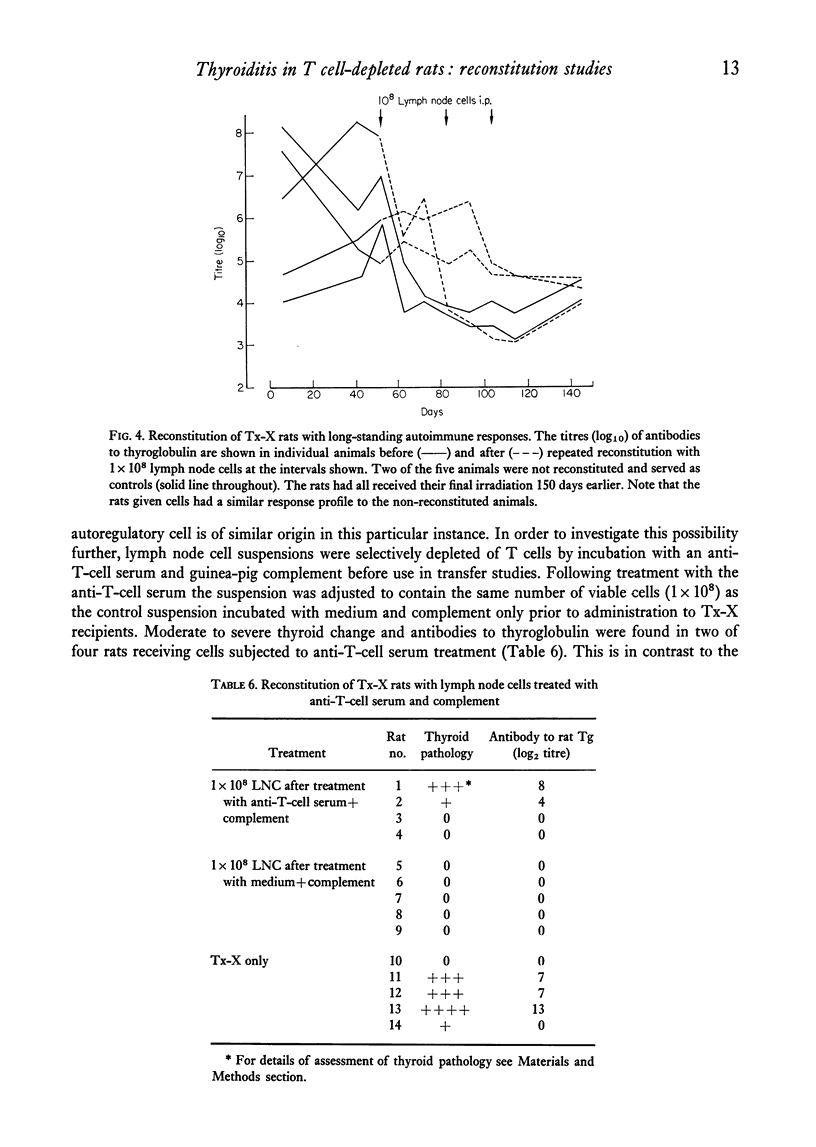
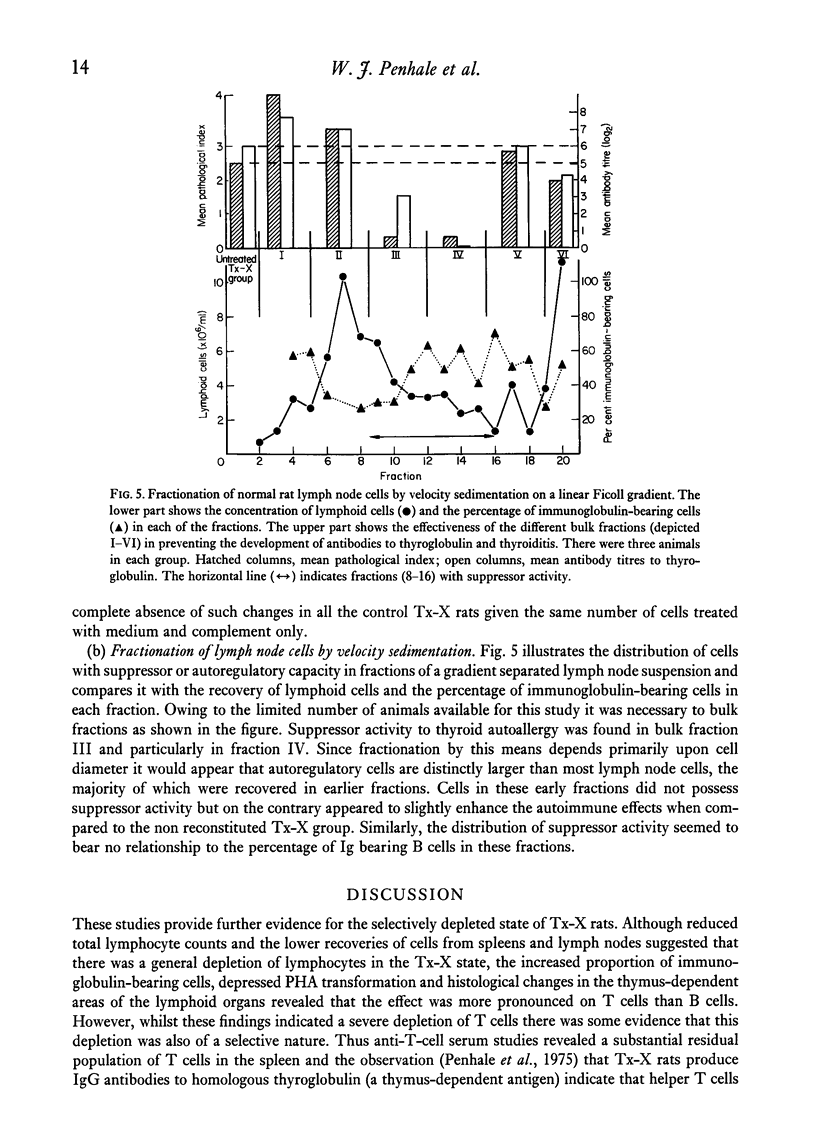
Qualititive, quantitative and functional differences were found in lymphoid cells of female thymectomized and irradiated (Tx-X) PVG/c strain rats as compared to normal females of the same strain. Tx-X rats were lymphopenic and had reduced numbers of cells within spleen and cervical lymph nodes, depressed transformation responses of peripheral blood lymphocytes to PHA and lower percentage killing of their spleen cells by anti-T-cell serum and complement. There was an increased percentage of immunoglobulin-bearing cells in the lymph nodes. Reconstitution of Tx-X rats by the intravenous route using syngeneic lymph node cells, spleen cells or thymocytes abrogated the autoimmune responses to thyroid components generally observed in this state. Lymph node and spleen cells, but not thymocytes, also prevented thyroid changes when given intraperitoneally. In contrast, bone marrow cells appeared to give enhanced responses. Quntitative studies showed that the relative proportions of the suppressor or autoregulatory cells in various lymphoid tissues were lymph node greater than spleen greater than thymus. Complete abrogation of the autoimmune responses was possible only when cells were administered within a short time of final dose of irradiation and moderate thyroid change was again seen if transfer was delayed for 14 days post-irradiation. At 28 days reconstitution had no influence on the development of the autoimmune responses. Preliminary characterization studies using an anti-T-cell serum and fractionation of lymph node cells on a linear Ficoll gradient suggested that autoregulatory cell is a large T cell.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gershon R. K., Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970 May;18(5):723–737. [PMC free article] [PubMed] [Google Scholar]
- Greaves M. F., Tursi A., Playfair J. H., Torrigiani G., Zamir R., Roitt I. M. Immunosuppressive potency and in-vitro activity of antilymphocyte globulin. Lancet. 1969 Jan 11;1(7585):68–72. doi: 10.1016/s0140-6736(69)91089-7. [DOI] [PubMed] [Google Scholar]
- Greenberg A. H., Shen L., Roitt I. M. Characterization of the antibody-dependent cytotoxic cell. A non-phagocytic monocyte? Clin Exp Immunol. 1973 Oct;15(2):251–259. [PMC free article] [PubMed] [Google Scholar]
- Gyöngyössy M. I., Playfair J. H. Rosette formation by mouse lymphocytes. I. Demonstration by indirect immunofluorescence of T cells binding sheep erythrocytes. Clin Exp Immunol. 1974 Oct;18(2):169–176. [PMC free article] [PubMed] [Google Scholar]
- Ha T. Y., Waksman B. H., Treffers H. P. The thymic suppressor cell. I. Separation of subpopulations with suppressor activity. J Exp Med. 1974 Jan 1;139(1):13–23. doi: 10.1084/jem.139.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzenberg L. A., Chan E. L., Ravitch M. M., Riblet R. J., Herzenberg L. A. Active suppression of immunoglobulin allotype synthesis. 3. Identification of T cells as responsible for suppression by cells from spleen, thymus, lymph node, and bone marrow. J Exp Med. 1973 Jun 1;137(6):1311–1324. doi: 10.1084/jem.137.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp J. A., Pierce C. W., Schlossman S., Benacerraf B. Genetic control of immune responses in vitro. V. Stimulation of suppressor T cells in nonresponder mice by the terpolymer L-glutamic acid 60-L-alanine 30-L-tyrosine 10 (GAT). J Exp Med. 1974 Sep 1;140(3):648–659. doi: 10.1084/jem.140.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel R. S., Eidinger D. Enhanced immune responsiveness to a thymus-independent antigen early after adult thymectomy: evidence for short-lived inhibitory thymus-derived cells. Eur J Immunol. 1972 Apr;2(2):114–118. doi: 10.1002/eji.1830020204. [DOI] [PubMed] [Google Scholar]
- Okumura K., Tada T. Regulation of homocytotropic antibody formation in the rat. VI. Inhibitory effect of thymocytes on the homocytotropic antibody response. J Immunol. 1971 Dec;107(6):1682–1689. [PubMed] [Google Scholar]
- Penhale W. J., Farmer A., Irvine W. J. Thyroiditis in T cell-depleted rats. Influence of strain, radiation dose, adjuvants and antilymphocyte serum. Clin Exp Immunol. 1975 Sep;21(3):362–375. [PMC free article] [PubMed] [Google Scholar]
- Penhale W. J., Farmer A., McKenna R. P., Irvine W. J. Spontaneous thyroiditis in thymectomized and irradiated Wistar rats. Clin Exp Immunol. 1973 Oct;15(2):225–236. [PMC free article] [PubMed] [Google Scholar]
- Tada T., Taniguchi M., Okumura K. Regulation of homocytotropic antibody formation in the rat. II. Effect of X-irradiation. J Immunol. 1971 Apr;106(4):1012–1018. [PubMed] [Google Scholar]



