Abstract
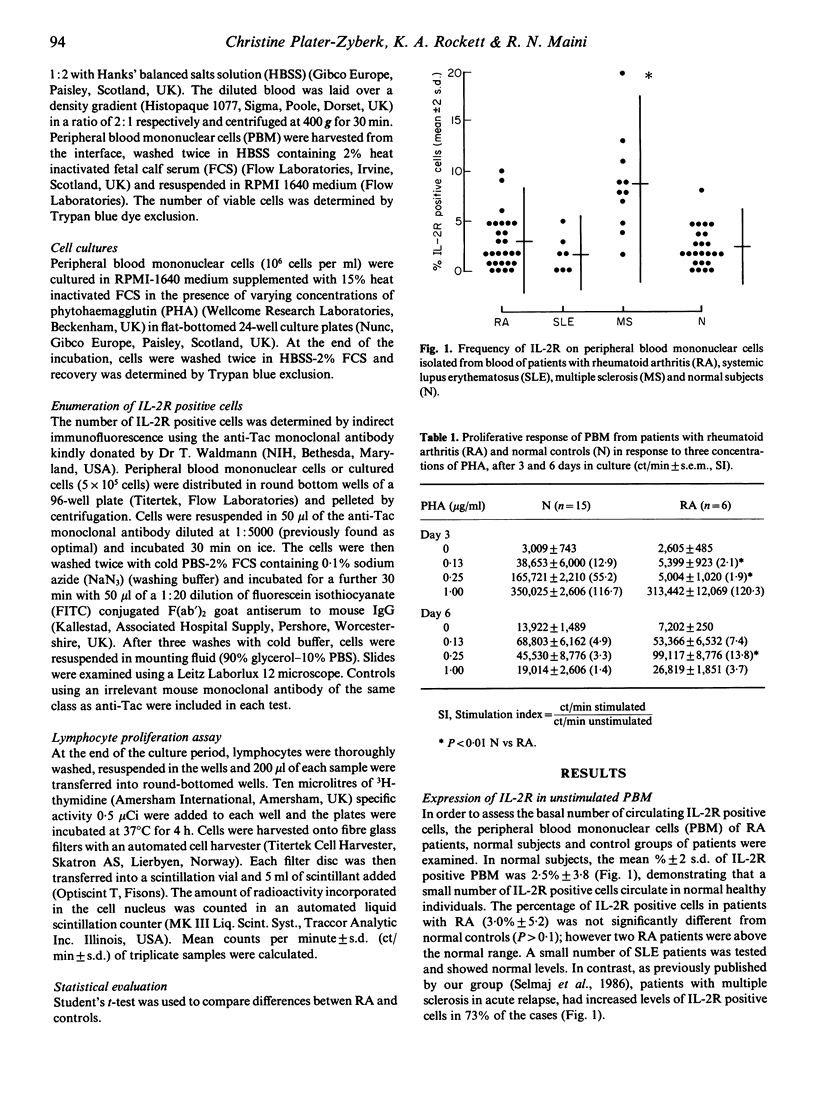
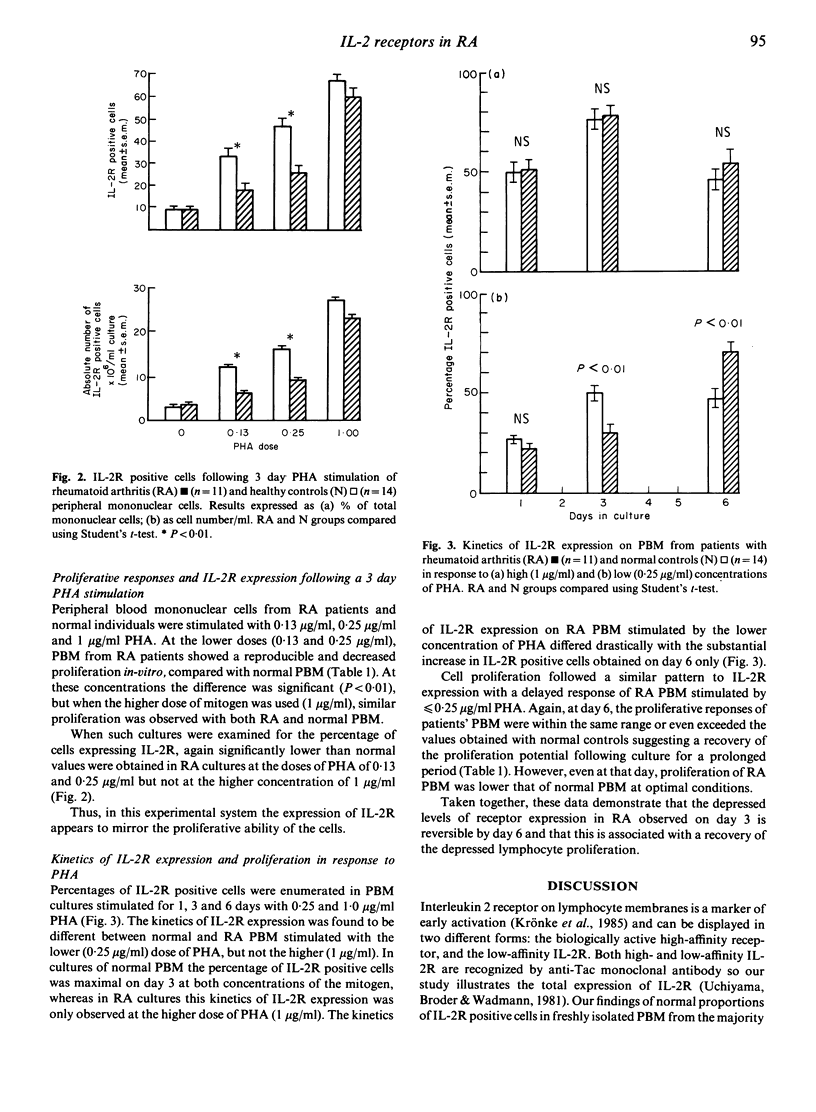
Peripheral blood mononuclear cells (PBM) from 11 patients with rheumatoid arthritis (RA) stimulated with 0.13 and 0.25 microgram/ml phytohaemagglutinin (PHA) for 3 days showed a depressed expression of interleukin 2 receptor (IL-2R) when compared with 14 normal controls (P less than 0.01). At these two doses of PHA a depressed lymphocyte proliferative response was also observed (P less than 0.01). However the kinetics of the response of the RA group differed from those of the control group. Whereas by day 6 IL-2R expression and lymphocyte proliferation in the control group was decreased compared with day 3, responses of the RA cells were increased. Following stimulation with the higher dose of PHA (1 microgram/ml) the kinetics of lymphocyte proliferation and IL-2R expression were equivalent in control and RA cultures. These results demonstrate that the impaired IL-2R expression and lymphocyte proliferation observed with sub-optimally stimulated PBM from RA patients is spontaneously reversed during prolonged culture and is consistent with the hypothesis that there is a lack of available IL-2 in the early stages of culture.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcocer-Varela J., Alarcón-Segovia D. Decreased production of and response to interleukin-2 by cultured lymphocytes from patients with systemic lupus erythematosus. J Clin Invest. 1982 Jun;69(6):1388–1392. doi: 10.1172/JCI110579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman A., Theofilopoulos A. N., Weiner R., Katz D. H., Dixon F. J. Analysis of T cell function in autoimmune murine strains. Defects in production and responsiveness to interleukin 2. J Exp Med. 1981 Sep 1;154(3):791–808. doi: 10.1084/jem.154.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester G. R., Jahn B., Gramatzki M., Zacher J., Kalden J. R. Activated T cells in vivo and in vitro: divergence in expression of Tac and Ia antigens in the nonblastoid small T cells of inflammation and normal T cells activated in vitro. J Immunol. 1984 Sep;133(3):1230–1234. [PubMed] [Google Scholar]
- Burmester G. R., Yu D. T., Irani A. M., Kunkel H. G., Winchester R. J. Ia+ T cells in synovial fluid and tissues of patients with rheumatoid arthritis. Arthritis Rheum. 1981 Nov;24(11):1370–1376. doi: 10.1002/art.1780241106. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. The interleukin-2 T-cell system: a new cell growth model. Science. 1984 Jun 22;224(4655):1312–1316. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. Transient expression of interleukin 2 receptors. Consequences for T cell growth. J Exp Med. 1983 Dec 1;158(6):1895–1911. doi: 10.1084/jem.158.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combe B., Pope R. M., Fischbach M., Darnell B., Baron S., Talal N. Interleukin-2 in rheumatoid arthritis: production of and response to interleukin-2 in rheumatoid synovial fluid, synovial tissue and peripheral blood. Clin Exp Immunol. 1985 Mar;59(3):520–528. [PMC free article] [PubMed] [Google Scholar]
- DIAGNOSTIC criteria for rheumatoid arthritis: 1958 revision by a committee of the American Rheumatism Association. Ann Rheum Dis. 1959 Mar;18(1):49–53. [PMC free article] [PubMed] [Google Scholar]
- Dauphinée M. J., Kipper S. B., Wofsy D., Talal N. Interleukin 2 deficiency is a common feature of autoimmune mice. J Immunol. 1981 Dec;127(6):2483–2487. [PubMed] [Google Scholar]
- Emery P., Panayi G. S., Nouri A. M. Interleukin-2 reverses deficient cell-mediated immune responses in rheumatoid arthritis. Clin Exp Immunol. 1984 Jul;57(1):123–129. [PMC free article] [PubMed] [Google Scholar]
- Horwitz D. A., Garrett M. A. Lymphocyte reactivity to mitogens in subjects with systemic lupus erythematosus, rheumatoid arthritis and scleroderma. Clin Exp Immunol. 1977 Jan;27(1):92–99. [PMC free article] [PubMed] [Google Scholar]
- Huang Y. P., Miescher P. A., Zubler R. H. The interleukin 2 secretion defect in vitro in systemic lupus erythematosus is reversible in rested cultured T cells. J Immunol. 1986 Dec 1;137(11):3515–3520. [PubMed] [Google Scholar]
- Kermani-Arab V., Hirji K., Ahmed A. R., Fahey J. L. Deficiency of interleukin-2 production and interleukin-2 receptor expression on peripheral blood leukocytes after phytohemagglutinin stimulation in pemphigus. J Invest Dermatol. 1984 Aug;83(2):101–104. doi: 10.1111/1523-1747.ep12263194. [DOI] [PubMed] [Google Scholar]
- Kluin-Nelemans H. C., van der Linden J. A., Gmelig Meyling F. H., Schuurman H. J. HLA-DR positive T lymphocytes in blood and synovial fluid in rheumatoid arthritis. J Rheumatol. 1984 Jun;11(3):272–276. [PubMed] [Google Scholar]
- Krönke M., Leonard W. J., Depper J. M., Greene W. C. Sequential expression of genes involved in human T lymphocyte growth and differentiation. J Exp Med. 1985 Jun 1;161(6):1593–1598. doi: 10.1084/jem.161.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M., Tsoukas C. D., Robinson C. A., Dinarello C. A., Carson D. A., Vaughan J. H. Basis for defective responses of rheumatoid arthritis synovial fluid lymphocytes to anti-CD3 (T3) antibodies. J Clin Invest. 1986 Sep;78(3):713–721. doi: 10.1172/JCI112631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec P., Kashiwado T., Ziff M. Inhibitor of interleukin-2 in rheumatoid synovial fluid. Arthritis Rheum. 1987 Feb;30(2):121–129. doi: 10.1002/art.1780300201. [DOI] [PubMed] [Google Scholar]
- Miyasaka N., Nakamura T., Russell I. J., Talal N. Interleukin 2 deficiencies in rheumatoid arthritis and systemic lupus erythematosus. Clin Immunol Immunopathol. 1984 Apr;31(1):109–117. doi: 10.1016/0090-1229(84)90195-8. [DOI] [PubMed] [Google Scholar]
- Murakawa Y., Takada S., Ueda Y., Suzuki N., Hoshino T., Sakane T. Characterization of T lymphocyte subpopulations responsible for deficient interleukin 2 activity in patients with systemic lupus erythematosus. J Immunol. 1985 Jan;134(1):187–195. [PubMed] [Google Scholar]
- Poulter L. W., Duke O., Panayi G. S., Hobbs S., Raftery M. J., Janossy G. Activated T lymphocytes of the synovial membrane in rheumatoid arthritis and other arthropathies. Scand J Immunol. 1985 Dec;22(6):683–690. doi: 10.1111/j.1365-3083.1985.tb01931.x. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Munck A., Smith K. A. T cell growth factor receptors. Quantitation, specificity, and biological relevance. J Exp Med. 1981 Nov 1;154(5):1455–1474. doi: 10.1084/jem.154.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon M., Bacon P. A., Symmons D. P., Blann A. D. Transferrin receptor bearing cells in the peripheral blood of patients with rheumatoid arthritis. Clin Exp Immunol. 1985 Nov;62(2):346–352. [PMC free article] [PubMed] [Google Scholar]
- Selmaj K., Plater-Zyberk C., Rockett K. A., Maini R. N., Alam R., Perkin G. D., Rose F. C. Multiple sclerosis: increased expression of interleukin-2 receptors on lymphocytes. Neurology. 1986 Oct;36(10):1392–1395. doi: 10.1212/wnl.36.10.1392. [DOI] [PubMed] [Google Scholar]
- Silver R. M., Redelman D., Zvaifler N. J. Studies of rheumatoid synovial fluid lymphocytes. II. A comparison of their behavior with blood mononuclear cells in the autologous mixed lymphocyte reaction and response to TCGF. Clin Immunol Immunopathol. 1983 Apr;27(1):15–27. doi: 10.1016/0090-1229(83)90052-1. [DOI] [PubMed] [Google Scholar]
- Silverman H. A., Johnson J. S., Vaughan J. H., McGlamory J. C. Altered lymphocyte reactivity in rheumatoid arthritis. Arthritis Rheum. 1976 May-Jun;19(3):509–515. doi: 10.1002/art.1780190301. [DOI] [PubMed] [Google Scholar]
- Smith K. A. Interleukin 2. Annu Rev Immunol. 1984;2:319–333. doi: 10.1146/annurev.iy.02.040184.001535. [DOI] [PubMed] [Google Scholar]
- Smith K. A. T-cell growth factor. Immunol Rev. 1980;51:337–357. doi: 10.1111/j.1600-065x.1980.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Wolf R. E. Hyporesponsiveness of lymphocytes to virus antigens in rheumatoid arthritis. Arthritis Rheum. 1978 Mar;21(2):238–242. doi: 10.1002/art.1780210211. [DOI] [PubMed] [Google Scholar]
- Yu D. T., Winchester R. J., Fu S. M., Gibofsky A., Ko H. S., Kunkel H. G. Peripheral blood Ia-positive T cells. Increases in certain diseases and after immunization. J Exp Med. 1980 Jan 1;151(1):91–100. doi: 10.1084/jem.151.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]



