Abstract
Background
At present, node-negative, high-risk breast cancer patients cannot be identified with sufficient accuracy. Consequently, further strong prognostic factors are needed.
Methods
Among 181 node-negative breast cancer (NNBC) patients, c-myc and HER-2/neu oncogenes were identified prospectively using double differential PCR. The possible impact of amplification of those oncogenes on disease-free survival (DFS) and overall survival was examined. Furthermore, the possible effects of adjuvant therapies on rate of recurrence and mortality in oncogene-amplified NNBC patients were investigated.
Results
The prevalence rates for amplification of c-myc and HER-2/neu were 21.5% and 30.4%, respectively. On univariate analysis, c-myc-amplified NNBCs were associated with significantly shorter DFS at 36 months after the initial diagnosis (85.3% versus 97.3%). As compared with nonamplified cancers, HER-2/neu-amplified NNBCs did not exhibit any significant differences after 36 months and 95 months. Multivariate analysis indicated that c-myc amplification and tumour size, in contrast to HER-2/neu amplification, oestrogen receptor status, grading and age, were the only independent parameters for DFS. During the period of observation, we found no evidence for an impact of amplification of the oncogenes on overall survival in all cases. With respect to various adjuvant systemic therapies such as chemotherapy (cyclophosphamide, methotrexate, 5-fluorouracil; fluorouracil, epirubicin, cyclophosphamide) and endocrine therapy (tamoxifen), no significant differences were identified in oncogene-amplified NNBC patients in terms of DFS and overall survival. However, those c-myc-amplified NNBC patients who did not receive adjuvant systemic therapy exhibited significantly shorter DFS and overall survival as compared with c-myc-nonamplified patients.
Conclusion
C-myc amplification appears to be a strong prognostic marker with which to predict early recurrence in NNBC patients. C-myc-amplified NNBC patients without adjuvant systemic therapy experienced shorter DFS and overall survival.
Keywords: amplification of oncogenes, c-myc, disease-free survival, HER-2/neu, mortality, node-negative breast cancer, overall survival, recurrence
Introduction
Breast cancer is the most common form of cancer in women from developed countries. Axillary lymph node status is widely accepted as an important parameter for assessing prognosis in breast cancer patients. However, recurrence and death also occur in patients with node-negative breast cancer (NNBC). A recurrence rate of 30% may be expected during the first 5 years after diagnosis. Prognostic factors such as tumour size, tumour grading, hormone receptor status, age, histology, ploidy and proliferation index are used to define subgroups of high-risk NNBC patients [1-8]. Despite the availability of these prognostic markers, high-risk NNBC patients cannot be identified with sufficient accuracy. This has led to a search for new and possibly stronger prognostic markers in order to define new subgroups and to facilitate decision-making with respect to appropriate therapy [2,3,9].
Numerous reports have described the correlation between amplification of oncogenes and its impact on the course of breast cancer disease [4-7],[10-32]. In a meta-analysis in which 29 studies were evaluated [33], c-myc amplification exhibited significant but weak associations with tumour grade, lymph node metastasis, negative progesterone receptor status and postmenopausal status. Furthermore c-myc amplification was significantly associated with risk for recurrence and death. However, studies in recent years have further shown that the c-myc gene participates in most aspects of cellular function, including replication, growth, metabolism, differentiation and apoptosis [34]. Amplification of the oncogene HER-2/neu has also been shown to be indicative of poor prognosis in breast cancer. Studies revealed that the prognostic effect of HER-2/neu is stronger for survival than for recurrence [16,19,20,22,27,29]. Furthermore, increased HER-2/neu levels in primary tumours were associated with a poor response to endocrine therapy [5,12,15,32].
A drawback of many studies of oncogenes in human breast cancer is that usually only one oncogene was evaluated. Based on a series of unselected cases, in the present study we examined the possible influence of the amplification of the oncogenes c-myc and HER-2/neu on disease-free survival (DFS) and overall survival (OS). Furthermore we studied whether adjuvant therapies such as chemotherapy and endocrine treatment or no treatment at all had any impact on DFS and OS among oncogene-amplified NNBC patients [1-3,8,9,32,35-38].
Patients and methods
Among 181 NNBC patients who had undergone breast-conserving therapy or modified mastectomy combined with axillary lymphadenectomy of level 1 and 2 (at least 10 lymph nodes per patient had been removed), c-myc and HER-2/neu oncogenes were assessed prospectively using double differential PCR.
Table 1 shows some clinical, histological and molecular parameters. The median follow-up period was 42 months. Postoperatively, the following therapies were administered in addition to radiotherapy: tamoxifen 20 mg/day for 5 years in 54.7% of patients; and chemotherapy (six cycles of cyclophosphamide, methotrexate, 5-fluorouracil [CMF] or four cycles of fluorouracil, epirubicin, cyclophosphamide [FEC]) in 33.7% of patients. Of the NNBC patients, 11.6% did not receive any further systemic therapy.
Table 1.
Clinical, histological and molecular parameters of 181 node-negative breast cancer patients
| Parameter | Number of patients (%) |
| Tumour size | |
| T1 | 118 (65.2) |
| T2 | 54 (29.8) |
| T3/4 | 9 (5.0) |
| Age (years) | |
| <40 | 14 (7.7) |
| 40–55 | 46 (25.4) |
| >55 | 121 (66.9) |
| Histopathological grading | |
| G1 | 5 (2.8) |
| G2 | 105 (58) |
| G3 | 71 (39.2) |
| ER status | |
| ER negative | 64 (35.4) |
| ER positive | 115 (63.5) |
| Unknown | 2 (1.1) |
| PR status | |
| PR negative | 66 (36.5) |
| PR positive | 112 (61.9) |
| Unknown | 3 (1.7) |
| Oncogenes | |
| C-myc nonamplified | 142 (78.5) |
| C-myc amplified | 39 (21.5) |
| HER-2/neu nonamplified | 126 (69.6) |
| HER-2/neu amplified | 55 (30.4) |
| C-myc and HER-2/neu coamplified | 22 (12.2) |
ER, oestrogen receptor; PR, progesterone receptor.
After tissue preparation, malignant and normal tissues were kept fresh and transported to the pathologist (U.B.). The pathologist dissected samples for assessment of oncogenes and hormone receptors. A positive receptor status was defined as the presence of more than 10 fmol/mg cytosol protein. The histopathological grading was performed according to the method of Bloom and Richardson [39]. Lymph node sections were stained with haemotoxylin and eosin; immunohistochemical investigations were not performed. The tumour tissues were stored at -70°C and the DNA was isolated using the Fast Prep System (Bio 101 Savant, Savant Instruments, Inc., Holbrook, NY, USA). Less than 200 mg (10–200 mg) tissue was homogenized using FastDNA Kit in the Fast Prep Machine. After preparing the DNA, the content was measured and the isolated DNA was stored at -20°C. General details for the double differential PCR technique, reproducibility and clinical significance were described previously [17,18,28,40]. A cutoff point of more than 2.0 average gene copy number (AGCN) was considered to be positive for HER-2/neu amplification [40]. To calculate the cutoff point for c-myc amplification, all NNBC patients were subjected to Classification and Regression Trees (CART) analysis. A cutoff value of 2.1 AGCN for c-myc oncogene led to the best distinction between patients. Fig. 1 shows the CART analysis for all c-myc values [41].
Figure 1.
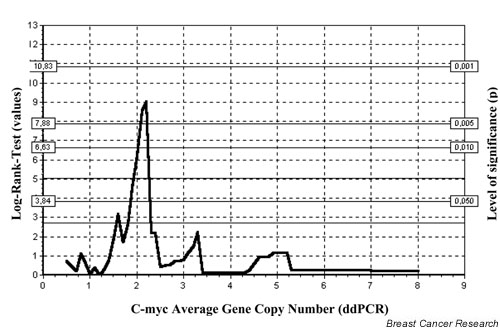
Classification and Regression Trees (CART) analysis of c-myc among 181 node-negative breast cancer patients. dd, double differential.
Results were evaluated using the SPSS system (SPSS GmbH Software, Munich, Germany). The monoparametric survival curves were determined using the Kaplan–Meier method in order to estimate the impacts of intratumoural c-myc and HER-2/neu oncogene amplification on DFS and OS. Statistical deviations were defined using the log-rank test. Recurrence of disease was found at the following locations: local (n = 2), contralateral (n = 1), axilla (n = 2), lung (n = 1), brain (n = 1), liver (n = 1) and skin (n = 1). During the period of observation, 14 patients died. In order to derive relevant information regarding the effects of oncogene amplification on the course of breast cancer disease, the accumulated values were determined after postoperative periods of 36 and 95 months. We applied the multivariate Cox model to enable us to identify independently predictive parameters [42]. Parameters considered included the oncogenes c-myc and HER-2/neu, tumour size, histopathological grading, oestrogen receptor status and age (< 40 or ≥ 40 years). P < 0.05 was considered statistically significant.
Results
The prevalence rates for amplification of c-myc and HER-2/neu were 21.5% and 30.4%, respectively. Coamplification was found in 22 patients (12.2%; Table 1). Among those patients with c-myc amplification five recurrences were identified (5/39 [12.8%]), and among those with HER-2/neu amplification two recurrences (2/55 [3.6%]) were identified. Among those patients in whom neither c-myc nor HER-2/neu were amplified four recurrences were found (4/109 [3.6%]), and among those with coamplification of both oncogenes two recurrences (2/22 [9.1%]) were observed. Four patients with c-myc amplification (4/39 [10.3%]) and three patients with HER-2/neu amplification (3/55 [5.5%]) died. Nine patients who lacked amplification of either oncogene (9/109 [8.3%]) and two patients with coamplification (2/22 [9.1%]) died.
Using univariate analysis, c-myc-amplified cancers were associated with a significantly lower DFS of 85.3%, as compared with 97.3% (P = 0.0290) among c-myc-non-amplified breast cancers. Ninety-five months after diagnosis (operation), the estimated DFS of c-myc-amplified cancer patients was only 85.3%, as compared with 95.6% among c-myc-nonamplified patients (P = 0.0079). Comparison of nonamplified cancers with HER-2/neu-amplified cancers did not reveal any significant differences with regard to DFS (Fig. 2).
Figure 2.
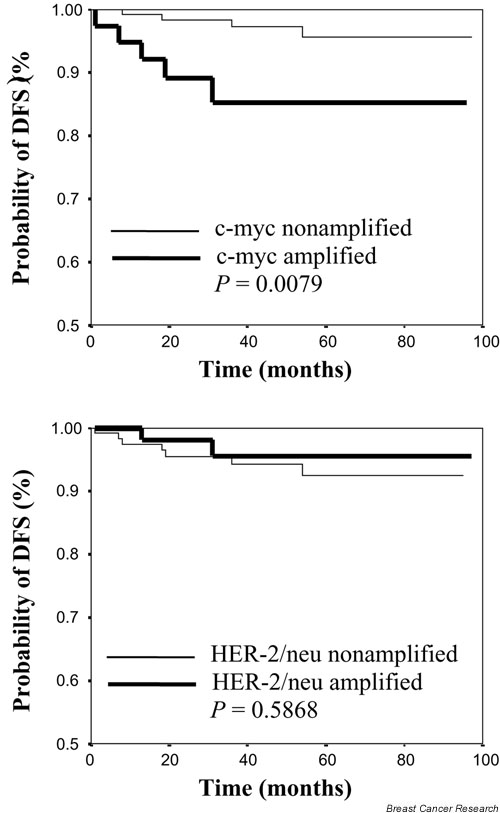
Kaplan–Meier estimation of disease-free survival (DFS) among 181 node-negative breast cancer patients.
Multivariate analysis revealed that c-myc amplification and tumour size, in contrast to oestrogen receptor status, grading and age, were the only independent parameters impacting on DFS (Table 2). With regard to OS, no independent parameters were identified among the prognostic markers referred to above.
Table 2.
Tumour parameters and disease-free survival in 181 node-negative breast cancer patients in univariate and multivariate analyses
| DFS after 36 months | DFS after 95 months | |||
| Parameter | Univariate P value | Multivariate P value | Univariate P value | Multivariate P value |
| Tumour size | 0.0223* | 0.024* | 0.0047* | 0.002* |
| c-myc | 0.0290* | 0.013* | 0.0079* | 0.004* |
| HER-2/neu | 0.2943 | 0.252 | 0.5868 | 0.166 |
| Oestrogen receptor | 0.6797 | 0.192 | 0.5363 | 0.622 |
| Tumour grade | 0.0998 | 0.128 | 0.0255* | 0.663 |
| Age < 40, ≥ 40 | 0.2330 | 0.965 | 0.0920 | 0.717 |
*P < 0.05.
Regarding various adjuvant systemic therapies, such as chemotherapy (CMF, FEC) and endocrine therapy (tamoxifen), no significant differences were observed with respect to DFS and OS in c-myc-amplified and HER-2/neu-amplified NNBC patients in comparison with non-amplified patients. However, significantly shorter DFS and OS were observed among c-myc-amplified patients who did not receive systemic adjuvant therapy. No corresponding associations were found among HER-2/neu-amplified NNBC patients as compared with nonamplified patients (Tables 3 and 4).
Table 3.
Recurrences and disease-free survival in 181 node-negative breast cancer patients after different adjuvant systemic therapies or no therapy
| Subgroup | n | Recurrence (n [%]) | DFS (P) | |
| Chemotherapy (n = 61) | ||||
| c-myc | Amplified | 19 | 3 (15.8) | 0.1748 |
| Nonamplified | 42 | 2 (4.8) | ||
| HER-2/neu | Amplified | 26 | 1 (3.8) | 0.2552 |
| Nonamplified | 35 | 4 (11.4) | ||
| Endocrine therapy (n= 99) | ||||
| c-myc | Amplified | 15 | 1 (6.7) | 0.3325 |
| Nonamplified | 84 | 2 (2.4) | ||
| HER-2/neu | Amplified | 22 | 1 (4.5) | 0.6641 |
| Nonamplified | 77 | 2 (2.6) | ||
| No therapy (n = 21) | ||||
| c-myc | Amplified | 5 | 1 (20.0) | 0.0209* |
| Nonamplified | 16 | 0 (0) | ||
| HER-2/neu | Amplified | 7 | 0 (0) | 0.4969 |
| Nonamplified | 14 | 1 (7.1) | ||
*P < 0.05 (log-rank test). DFS, disease-free survival.
Table 4.
Mortality and overall survival in 181 node-negative breast cancer patients after different adjuvant systemic therapies or no therapy
| Subgroup | n | Mortality (n [%]) | OS (P) | |
| Chemotherapy (n = 61) | ||||
| c-myc | Amplified | 19 | 1 (5.3) | 0.7442 |
| Nonamplified | 42 | 3 (7.1) | ||
| HER-2/neu | Amplified | 26 | 0 (0.0) | 0.1042 |
| Nonamplified | 35 | 4 (11.4) | ||
| Endocrine therapy (n= 99) | ||||
| c-myc | Amplified | 15 | 1 (6.7) | 0.9846 |
| Nonamplified | 84 | 6 (7.1) | ||
| HER-2/neu | Amplified | 22 | 2 (9.1) | 0.8307 |
| Nonamplified | 77 | 5 (6.5) | ||
| No therapy (n = 21) | ||||
| c-myc | Amplified | 5 | 2 (40.0) | 0.0098* |
| Nonamplified | 16 | 1 (6.3) | ||
| HER-2/neu | Amplified | 7 | 1 (14.3) | 0.5649 |
| Nonamplified | 14 | 2 (14.3) | ||
*P < 0.05 (log-rank test). OS, overall survival.
Discussion
Numerous clinical studies have proved axillary lymph node status to be the dominant factor for prognosis and prediction of DFS and OS in breast cancer patients. The fact of the matter is that even among NNBC patients 25–30% can be expected to progress or even die. The advantage conferred by adjuvant chemotherapy or endocrine therapy was examined in randomized studies [2,3,8,9,35-38]. Because adjuvant therapies may positively impact on outcome in only around 15%, the costs of these therapies make their routine application in all NNBC patients inappropriate [1]. For this reason, predictive factors that accurately define subgroups of NNBC patients who may benefit from adjuvant systemic therapy would be a great advantage.
In the present study the oncogenes c-myc and HER-2/neu were examined with regard to their ability to predict DFS, OS and rate of recurrence, as well as mortality. All patients were randomly selected from one department (Frauenklinik Klinikum Ibbenbueren, Ibbenbueren, Germany). C-myc amplification was identified in 21.5% and HER-2/neu amplification in 30.4%. Berns and coworkers [12-14] reported amplification of c-myc in 20% and of HER-2/neu in 24% using a standard southern blot technique. In a selected high-risk cohort of NNBC patients, Roux-Dosseto et al. [26] applied the same method and found prevalence rates for c-myc and HER-2/neu amplification of 25% and 31%, respectively. Those oncogenes were assessed in the present study using a double differential PCR technique [17,18,40,43]. Using this method, Brandt et al. [17] found c-myc to be amplified in 19.7% and HER-2/neu in 16.7% of breast cancers, without consideration of nodal status; coamplification of those oncogenes was present in 7%. In the present study, simultaneous amplification of both oncogenes was observed in 12.2%. As in the present investigation, Roux-Dosseto et al. [26] found that c-myc amplification among NNBC patients was significantly associated with earlier recurrence in univariate and multivariate analyses. However, HER-2/neu-amplified NNBC patients did not have the same outcome. Accordingly, c-myc amplification appears to be an independent prognostic marker, which has greater predictive power than does oestrogen receptor status and tumour grade. As early as 1992, Berns and coworkers [11,12,14] reported that patients with c-myc-amplified breast cancers had an unfavourable prognosis.
The first study to mention possible prognostic importance of HER-2/neu gene amplification was reported in 1987 by Slamon et al. [27]. That study included 187 patients with NNBC and node-positive breast cancer; by univariate and multivariate analyses, it revealed that HER-2/neu amplification correlated very closely with shorter DFS and OS in a subgroup of 87 node-positive patients. In 1993, in an analysis of 210 patients, Press et al. [7] found that amplification of HER-2/neu correlated with unfavourable prognosis with respect to DFS.
Attempts by other investigators to confirm these findings were met with various degrees of success. Some studies claimed that HER-2/neu status was an independent predictive factor in the case of breast cancer, whereas other studies could not confirm this [6,14,16,19,29,30]. It is certainly of great interest to the clinician that only two out of five studies including more than 100 patients and with a follow-up period of at least 3 years attributed some prognostic value to HER-2/neu amplification among patients with NNBC [6,7,16,19,28]. Almost all of the studies that dealt with HER-2/neu status and DFS, as well as OS, showed no benefit of this oncogene in the prognosis of NNBC patients. In a survey conducted by Ravdin and Chamness [24], only one [22] of 11 studies concerning immunohistochemical overexpression of HER-2/neu indicated a significant result with respect to OS in multivariate analysis.
In the present study, 19 out of 39 c-myc-amplified patients received chemotherapy, 15 patients received endocrine therapy and five patients received no further therapy. The greatest recurrence rates were noted in the group of patients who received no therapy (20%) and in those who received chemotherapy (16%). The lowest recurrence rate of 7% was seen in patients treated with tamoxifen. All patients who received endocrine therapy (n = 99) were characterized by positive oestrogen and/or progesterone receptor status. The proto-oncogene c-myc can be upregulated by oestrogen stimulation of hormone-dependent breast cancer cells. Endocrine therapy with the antioestrogen tamoxifen can mediate the downregulation of c-myc, culminating in cell cycle arrest [44]. Berns et al. [12] reported that 38% of patients with amplification of c-myc or with coamplification of c-myc and HER-2/neu profited from endocrine therapy. C-myc-amplified patients affected by metastatic disease showed a tendency toward longer DFS with endocrine therapy, as compared with shorter DFS following second-line chemotherapy. However, the minor rate of response after chemotherapy did not correlate with OS. The poorer responses to chemotherapy among patients with c-myc-amplified tumours in the present study (recurrence rate 16%) may be in agreement with experimental findings in erythroleukaemia cells that the degree of cis-platinum resistance correlated directly with the level of c-myc expression [45].
In the present study, 26 out of 55 HER-2/neu-amplified patients received chemotherapy, 22 patients received endocrine therapy and seven patients received no further therapy. In total, low recurrence rates of 3.8%, 4.5% and 0% were found in the above-mentioned therapy groups, respectively (Table 3). Compared with nonamplified patients administered the same therapies, no significant differences with regard to DFS and OS were observed. In contrast to that finding, c-myc-amplified patients not administered adjuvant systemic therapy exhibited a significantly shorter DFS and OS than did nonamplified patients who also received no adjuvant systemic therapy. However, the therapy groups analyzed in the present study are small, and therefore we recommend careful interpretation of the findings.
Higher recurrence rates or poor response to endocrine treatment with tamoxifen in patients with HER-2/neu-amplified hormone receptor positive tumours, as reported in studies in node-positive or metastatic breast cancer [12,15,32], were not observed in the present study. Although deregulated HER-2/neu activity can strongly stimulate cytoplasmic signalling pathways, which in turn impinge on c-myc at multiple levels causing its deregulated expression [46], this scenario does not appear to be active in NNBC because the recurrence rate of 22 c-myc and HER-2/neu coamplified patients was only 9%. However, among those patients in whom coamplification of c-myc and HER-2/neu was absent the recurrence rate was lower (4/109 [3.6%]). Differences in the numbers of recurrences and deaths can be accounted for by the fact that some deaths were not related to tumours.
Conclusion
C-myc amplification appears to represent a prognostic marker with which early recurrence may be predicted in NNBC patients. C-myc-amplified NNBC patients who were not administered adjuvant systemic therapy suffer shorter DFS and OS. C-myc could be used together with the tumour-associated protease urokinase-plasminogen activator and its inhibitor (i.e. plasminogen activator inhibitor-1), after further randomized studies have been conducted, to confirm whether NNBC patients should receive adjuvant systemic therapy [47-49].
Competing interests
None declared.
Abbreviations
AGCN = average gene copy number; CART = Classification and Regression Trees; CMF = cyclophosphamide, methotrexate, 5-fluorouracil; DFS = disease-free survival; NNBC = node-negative breast cancer; FEC = fluorouracil, epirubicin, cyclophosphamide; OS = overall survival; PCR = poly-merase chain reaction.
References
- McGuire W. Adjuvant therapy of node-negative breast cancer. N Engl J Med. 1989;320:525–527. doi: 10.1056/NEJM198902233200811. [DOI] [PubMed] [Google Scholar]
- Fisher B, Costantino J, Redmond C, Poisson R, Bowman D, Couture J, Dimitrov NV, Wolmark N, Wickerham DL, Fisher ER, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989;320:479–489. doi: 10.1056/NEJM198902233200802. [DOI] [PubMed] [Google Scholar]
- Fisher B, Redmond C, Dimitrov NV, Bowman D, Legault-Poisson S, Wickerman DL, Wolmark N, Fisher ER, Margolese R, Sutherland C, et al. A randomized clinical trial evaluating sequential methotrexate and fluorouracil in the treatment of patients with node-negative breast cancer. N Engl J Med. 1989;320:473–478. doi: 10.1056/NEJM198902233200801. [DOI] [PubMed] [Google Scholar]
- Allred DC, Clark GM, Tandon AK, Molina R, Tormey DC, Osborne CK, Gilchrist KW, Mansour EG, Abeloff M, Eudey L, McGuire W. HER-2/neu in node-negative breast cancer: prognostic significance of overexpression influenced by the presence of in situ carcinoma. J Clin Oncol. 1992;10:599–605. doi: 10.1200/JCO.1992.10.4.599. [DOI] [PubMed] [Google Scholar]
- Nicholson S, Wright S, Sainsbury JRC, Halcrow P, Kelly P, Angus B, Farndon JR, Harns AL. Epidermal growth factor receptor (EGFR) as a marker for poor prognosis in node-negative breast cancer patients: neu and tamoxifen failure. J Steroid Biochem. 1990;27:811–815. doi: 10.1016/0960-0760(90)90424-j. [DOI] [PubMed] [Google Scholar]
- Paterson MC, Dietrich KD, Danyluk J, Paterson AH, Lees AW, Jamil N, Hanson J, Jenkins H, Krause BE, McBlain WA, Slamon DJ, Fourney RM. Correlation between c-erb B-2 amplification and risk of recurrent disease in node-negative breast cancer. Cancer Res. 1991;51:4960–4970. [PubMed] [Google Scholar]
- Press MF, Pike MC, Chazin VR, Hung G, Udove JA, Markowicz M, Danyluk J, Godolphin W, Sliwowski M, Akita R, Paterson MC, Slamon DJ. Her-2/neu expression in node-negative breast cancer: direct tissue quantitation by computerized image analysis and association of overexpression with increased risk of recurrent disease. Cancer Res. 1993;53:4960–4970. [PubMed] [Google Scholar]
- The Ludwig Breast Cancer Study Group Prolonged disease-free survival after one course of perioperative adjuvant chemotherapy for node-negative breast cancer. N Engl J Med. 1989;320:491–495. doi: 10.1056/NEJM198902233200804. [DOI] [PubMed] [Google Scholar]
- Fisher B, Slack N, Katrych D, Wolmark N. Ten years follow up results of patients with carcinoma of the breast in a co-operative clinical trial evaluating surgical adjuvant chemotherapy. Surg Gynecol Obstet. 1975;140:528–534. [PubMed] [Google Scholar]
- Ali IU, Campbell G, Lidereau R, Callahan R. Lack of evidence for the prognostic significance of c-erb B-2 amplification. Oncogene Res. 1988;3:139–146. [PubMed] [Google Scholar]
- Berns PMJJ, Foekens JA, Van Putten WLJ, Van Staveren IL, Alexieva-Figusch J, Klijn JGM. Prognostic factors in human primary breast cancer: comparison of c-myc and HER2/neu amplification. J Steroid Biochem Mol Biol. 1992;43:13–19. doi: 10.1016/0960-0760(92)90182-i. [DOI] [PubMed] [Google Scholar]
- Berns PMJJ, Foekens JA, Van Staveren IL, Van Putten WLJ, De Koning HYWCM, Portengen Hm, Klijn JGM. Oncogene amplification and prognosis in breast cancer: relationship with systemic treatment. Gene. 1995;159:11–18. doi: 10.1016/0378-1119(94)00534-y. [DOI] [PubMed] [Google Scholar]
- Berns PMJJ, Foekens JA, Van Putten WLJ, Van Staveren IL, Alexieva-Figusch J, Klijn JGM. Prognostic factors in human primary breast cancer: comparison of c-myc and HER2/neu amplification. J Steroid Biochem Mol Biol. 1992;43:13–19. doi: 10.1016/0960-0760(92)90182-i. [DOI] [PubMed] [Google Scholar]
- Berns PMJJ, Klijn JGM, Van Putten WLJ, Van Staveren IL, Portengen H, Foekens JA. c-myc amplification is a better prognostic factor than HER2/neu amplification in primary breast cancer. Cancer Res. 1992;52:1107–1113. [PubMed] [Google Scholar]
- Bianco AR, Laurentils MD, Carlomagno C, Lauria R, Petrella G, Panico L, Pettinato G, Perrone F, Gallo C, Marinelli A, Placido SD. 20 year update of the Naples Gun trial of adjuvant breast cancer therapy: evidence of interaction between c-erb B-2 expression and tamoxifen efficacy [meeting abstract]. In Proceedings of the American Society of Clinical Oncology 34th Annual Meeting, Los Angeles, CA. 1998. p. 373.
- Borg A, Tandon AK, Sigurdsson H, Clark GM, Fernö M, Fuqua SA, Killander D, McGuire WL. c-erb B-2 amplification predicts poor survival in node-positive breast cancer. Cancer Res. 1990;50:4332–4337. [PubMed] [Google Scholar]
- Brandt B, Vogt U, Assmann G. Prognostische Relevanz der Amplifikation der Onkogene c-erb B-2 und c-myc beim Mammakarzinom [Prognostic relevance of amplifications of the oncogenes c-erb B-2 and c-myc in breast cancer]. Lab med. 1994;18:255. [Google Scholar]
- Brandt B, Vogt U, Schlotter CM, Jackisch C, Werkmeister R, Thomas M, von Eiff M, Bosse U, Assmann G, Zänker KS. Prognostic relevance of aberrations in the erbB oncogenes from breast, ovarian, oral and lung cancers: double-differential polymerase chain reaction (ddPCR) for clinical diagnosis. Gene. 1995;159:35–42. doi: 10.1016/0378-1119(94)00652-9. [DOI] [PubMed] [Google Scholar]
- Clark GM, McGuire WL. Follow-up study of HER-2/neu amplification in primary breast cancer. Cancer Res. 1991;51:944–948. [PubMed] [Google Scholar]
- Gusterson BA, Gelber RD, Goldhirsch A, Price KN, Save-Soderborgh J, Anbazhagan R, Styles J, Rudenstam CM, Golouh R, Reed R, et al. Prognostic importance of c-erb B-2 expression in breast cancer. International (Ludwig) Breast Cancer Study Group. J Clin Oncol. 1992;10:1049–1056. doi: 10.1200/JCO.1992.10.7.1049. [DOI] [PubMed] [Google Scholar]
- Henry JA, Hennessy C, Levett DL, Lennard TWJ, Westley BR, May FEB. int-2 amplification in breast cancer: Association with decreased survival and relationship to amplification of c-erb B-2 and c-myc. Int J Cancer. 1993;53:774–780. doi: 10.1002/ijc.2910530512. [DOI] [PubMed] [Google Scholar]
- Kallioniemi O-P, Holli K, Visakorpi T, Koivula T, Helin HH, Isola JJ. Association of c-erb B-2 protein over-expression with high rate of cell proliferation, increased risk of visceral metastasis and poor long-term survival in breast cancer. Int J Cancer. 1991;49:650–655. doi: 10.1002/ijc.2910490504. [DOI] [PubMed] [Google Scholar]
- Muss HB, Thor AD, Berry DA, Kute T, Liu ET, Koerner F, Cirrincione CT, Budman DR, Wood WC, Barcos M, Hernderson IC. c-erb B-2 expression and response to adjuvant therapy in women with node-positive breast cancer. N Engl J Med. 1994;330:1260–1266. doi: 10.1056/NEJM199405053301802. [DOI] [PubMed] [Google Scholar]
- Ravdin PR, Chamness GC. The c-erb B-2 proto-oncogene as a prognostic and predictive marker in breast cancer: a paradigm for the development of other macromolecular markers: a review. Gene. 1995;159:19–27. doi: 10.1016/0378-1119(94)00866-q. [DOI] [PubMed] [Google Scholar]
- Ravdin PM, Green S, Albain KS, Boucher V, Ingle J, Pritchard K, Shepard L, Davidson N, Hayes DF, Clark GM, Martino S, Osborne CK, Allred DC. Initial report of the SWOPG biological correlative study of c-erb B-2 expression as a predictor of outcome in a trial comparing adjuvant CAF-T with tamoxifen (T) alone [meeting abstract]. In Proceedings of the American Society of Clinical Oncology 34th Annual Meeting, Los Angeles, CA. 1998. p. 374.
- Roux-Dosseto M, Romain S, Dussault N, Desideri C, Piana L, Bonier P, Tubiana N, Martin PM. c-myc gene amplification in selected node-negative breast cancer patients correlates with high rate of early relapse. Eur J Cancer. 1992;28:1600–1604. doi: 10.1016/0959-8049(92)90050-c. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Clar GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the Her-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1998;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Tsuda H, Hirohashi S, Shimosato Y, Taaka Y, Hirota T, Tsugane S, Shiraishi M, Toyoshima K, Yamamoto T, Terada M. Immunohistochemical study on overexpression of c-erb B-2 protein in human breast cancer: its correlation with gene amplification and long-term survival of patients. Jpn J Cancer Res. 1990;81:327–332. doi: 10.1111/j.1349-7006.1990.tb02571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley J, Cooke T, Murray GD, Platt-Higgins A, George WD, Holt S, Myskov M, Spedding A, Barraclough BR, Rudland PS. The long term prognostic significance of c-erb B-2 in primary breast cancer. Br J Cancer. 1991;63:447–450. doi: 10.1038/bjc.1991.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C, Cairns J, Cantwell BJ, Cattan AR, Hall AG, Harris AL, Horne CH. Response to mitoxantrone in advanced breast cancer: correlation with expression of c-erb B2 protein and glutathione S-transferases. Br J Cancer. 1992;65:271–274. doi: 10.1038/bjc.1992.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C, Nicholson S, Angus B, Sainbury JR, Farndon J, Cairns J, Harris AL, Horne CH. Relationship between c-erb B-2 protein product expression and response to endocrine therapy in advanced breast cancer. Br J Cancer. 1992;65:118–121. doi: 10.1038/bjc.1992.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deming SL, Nass SJ, Dickson RB, Trock BJ. C-myc amplification in breast cancer: a meta-analysis of its occurrence and prognostic relevance. Br J Cancer. 2000;83:1688–1695. doi: 10.1054/bjoc.2000.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao DJ, Dickson RB. C-myc in breast cancer. Endocr Relat Cancer. 2000;7:143–164. doi: 10.1677/erc.0.0070143. [DOI] [PubMed] [Google Scholar]
- Anonymous Adjuvant tamoxifen in the management of operable breast cancer: the Scottish Trial. Report from the Breast Cancer Trials Committee, Scottish Cancer Trials Office (MRC), Edinburgh. Lancet. 1987;2:171–175. [PubMed] [Google Scholar]
- Bonnadonna G, Valagussa T, Zambetti M. Milan adjuvant trial for stage I-II breast cancer. In: Slamon SW, editor. In Adjuvant Chemotherapy of Cancer V. Orlando, Florida: Grune and Straton; 1987. pp. 211–221. [Google Scholar]
- Anonymous Controlled trial of tamoxifen as single adjuvant agent in management of early breast cancer. Analysis at six years by Nolvadex Adjuvant Trial Organisation. Lancet. 1985;1:836–840. [PubMed] [Google Scholar]
- Mansour EG, Gray R, Shatila AH, Osborne CK, Tormey DC, Gilchrist KW, Cooper MR, Falkson G. Efficacy of adjuvant chemotherapy for node-negative breast cancer. An Intergroup Study. N Engl J Med. 1989;320:485–490. doi: 10.1056/NEJM198902233200803. [DOI] [PubMed] [Google Scholar]
- Bloom HJG, Richardson WW. Histologic grading and prognosis in breast cancer. A study of 1409 cases which 359 have been followed for 15 years. Br J Cancer. 1957;11:359. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand B, Vogt U, Harms F, Bosse U, Zänker KS, Assmann G. Double-differential PCR for gene dosage estimation of erb B oncogenes in benign and cancer tissues and comparison to cellular DNA content. Gene. 1995;159:29–34. doi: 10.1016/0378-1119(94)00651-8. [DOI] [PubMed] [Google Scholar]
- Wassmann K. Prognostische Bedeutung DNA-Image-zytometrischer Parameter für das primäre Mammakarzinom [Prognostic significance of image cytometric DNA parameters for primary breast cancer]. Dissertation: University of Muenster, Muenster, Germany; 1999.
- Cox DR. Regression models and life tables (with discussion). J Royal Stat Soc. 1972;34:187–220. [Google Scholar]
- Vogt U, Bielawski K, Schlotter CM, Bosse U, Falkiewicz B, Podhajska AJ. Amplification of erb B-4 oncogene occurs less frequently than that of erb B-2 in primary human breast cancer. Gene. 1998;223:375–380. doi: 10.1016/s0378-1119(98)00454-5. [DOI] [PubMed] [Google Scholar]
- Carroll JS, Swarbrick A, Musgrove EA, Sutherland RL. Mechanisms of growth arrest by c-myc antisense oligonucleotides in MCF-7 breast cancer cells: implications for the antiproliferative effects of antiestrogens. Cancer Res. 2002;62:3126–3131. [PubMed] [Google Scholar]
- Sklar MD, Prochownik EV. Modulation of cis-platinum resistance in Friend erythroleukemia cells by c-myc. Cancer Res. 1991;51:2118–2123. [PubMed] [Google Scholar]
- Hynes NE, Lane HA. Myc and mammary cancer: Myc is a downstream effector of the ErbB2 receptor tyrosine kinase. J Mammary Gland Biol Neoplasia. 2001;6:141–150. doi: 10.1023/a:1009528918064. [DOI] [PubMed] [Google Scholar]
- Foekens JA, Look MP, Peters HA, van Putten WL, Portengen H, Klijn JGM. Urokinase-type plasminogen activator and its inhibitor PAI-1 predictor of poor response to tamoxifen therapy in recurrent breast cancer. J Natl Cancer Inst. 1995;87:751–756. doi: 10.1093/jnci/87.10.751. [DOI] [PubMed] [Google Scholar]
- Harbeck N, Dettmar P, Thomssen C, Henselmann B, Kuhn W, Ulm K, Jänicke F, Hofler H, Graeff H, Schmitt M. Prognostic impact of tumor biological factors on survival in node-negative breast cancer. Anticancer Res. 1998;18:2187–2197. [PubMed] [Google Scholar]
- Jänicke F, Schmitt M, Pacte L. Urokinase (uPA) and its inhibitor PAI-1 are strong independent prognostic factors in node-negative breast cancer. Breast Cancer Res Treat. 1992;24:195–208. doi: 10.1007/BF01833260. [DOI] [PubMed] [Google Scholar]


