Abstract
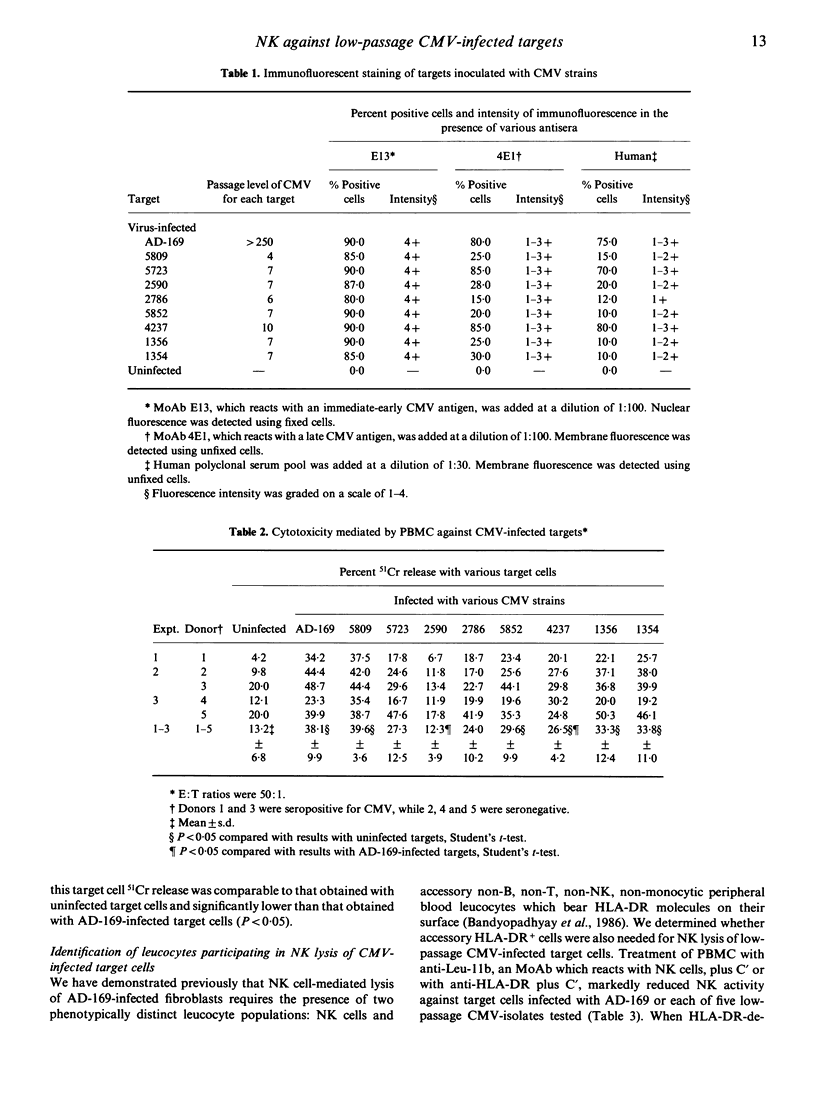
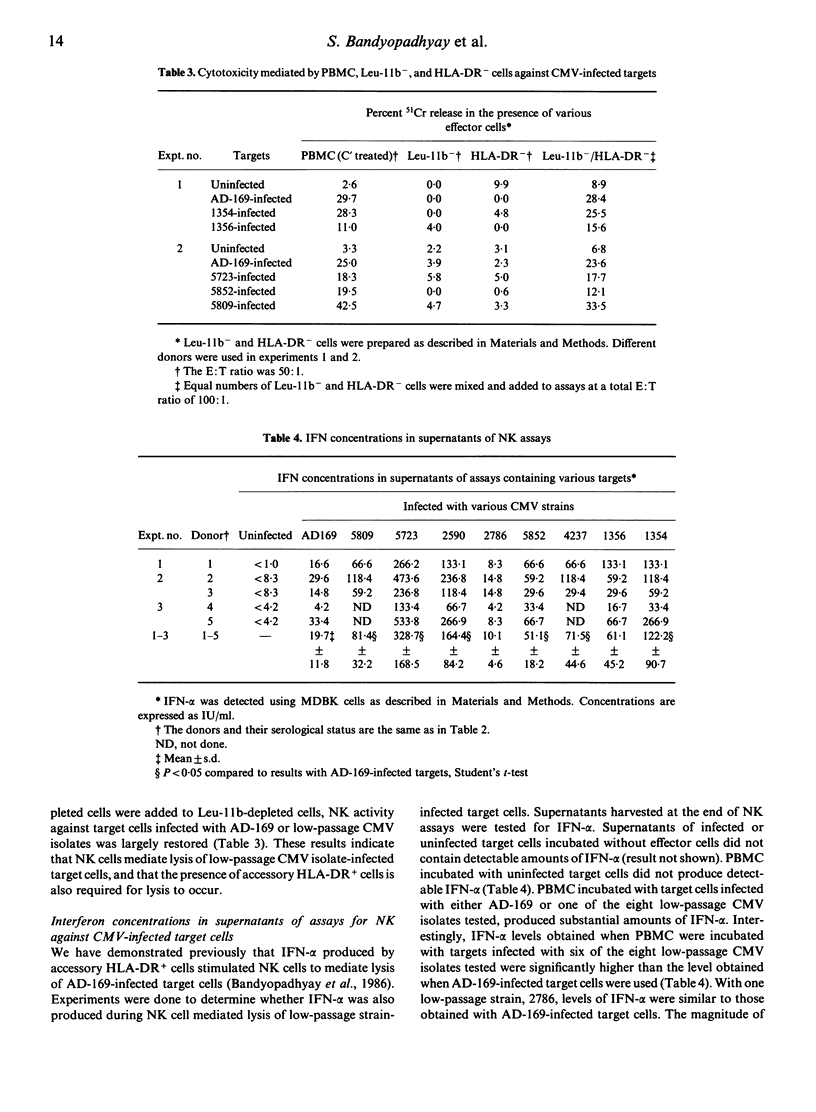
Fibroblasts infected with most low-passage clinical isolates of human cytomegalovirus (CMV) were as susceptible to lysis by human natural killer (NK) cells as high passage AD-169-infected fibroblasts. NK lysis occurred despite the absence of detectable CMV-specific late membrane antigen(s) on the majority of the target cells infected with most of the low passage strains. The magnitude of NK lysis of different CMV-infected target cells did not correlate with their ability to induce IFN-alpha. NK cell-mediated lysis of cells infected with low-passage clinical isolates of CMV required both NK cells and HLA-DR+ accessory cells, as previously shown for AD-169-infected target cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbeit R. D., Leary P. L., Levin M. J. Gamma interferon production by combinations of human peripheral blood lymphocytes, monocytes, and cultured macrophages. Infect Immun. 1982 Feb;35(2):383–390. doi: 10.1128/iai.35.2.383-390.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenzana-Seisdedos F., Virelizier J. L., Fiers W. Interferons as macrophage-activating factors. III. Preferential effects of interferon-gamma on the interleukin 1 secretory potential of fresh or aged human monocytes. J Immunol. 1985 Apr;134(4):2444–2448. [PubMed] [Google Scholar]
- Bandyopadhyay S., Perussia B., Trinchieri G., Miller D. S., Starr S. E. Requirement for HLA-DR+ accessory cells in natural killing of cytomegalovirus-infected fibroblasts. J Exp Med. 1986 Jul 1;164(1):180–195. doi: 10.1084/jem.164.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borysiewicz L. K., Graham S., Sissons J. G. Human natural killer cell lysis of virus-infected cells. Relationship to expression of the transferrin receptor. Eur J Immunol. 1986 Apr;16(4):405–411. doi: 10.1002/eji.1830160416. [DOI] [PubMed] [Google Scholar]
- Borysiewicz L. K., Rodgers B., Morris S., Graham S., Sissons J. G. Lysis of human cytomegalovirus infected fibroblasts by natural killer cells: demonstration of an interferon-independent component requiring expression of early viral proteins and characterization of effector cells. J Immunol. 1985 Apr;134(4):2695–2701. [PubMed] [Google Scholar]
- Furlini G., Gonczol E., Szokan G., Ianacone J., Plotkin S. A. Monoclonal antibodies directed to two groups of viral proteins neutralize human cytomegalovirus in vitro. Hybridoma. 1987 Jun;6(3):321–326. doi: 10.1089/hyb.1987.6.321. [DOI] [PubMed] [Google Scholar]
- Gresser I., Bandu M. T., Brouty-boye D., Tovey M. Pronounced antiviral activity of human interferon on bovine and porcine cells. Nature. 1974 Oct 11;251(5475):543–545. doi: 10.1038/251543a0. [DOI] [PubMed] [Google Scholar]
- Kettering J. D., Schmidt N. J., Gallo D., Lennette E. H. Anti-complement immunofluorescence test for antibodies to human cytomegalovirus. J Clin Microbiol. 1977 Dec;6(6):627–632. doi: 10.1128/jcm.6.6.627-632.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirmani N., Ginn R. K., Mittal K. K., Manischewitz J. F., Quinnan G. V., Jr Cytomegalovirus-specific cytotoxicity mediated by non-T lymphocytes from peripheral blood of normal volunteers. Infect Immun. 1981 Nov;34(2):441–447. doi: 10.1128/iai.34.2.441-447.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai K., Itoh K., Hinuma S., Tada M. Pretreatment of plastic Petri dishes with fetal calf serum. A simple method for macrophage isolation. J Immunol Methods. 1979;29(1):17–25. doi: 10.1016/0022-1759(79)90121-2. [DOI] [PubMed] [Google Scholar]
- Mazeron M. C., Berbar T., Guillemin M. C., Colimon R., Roseto A., Perol Y. Production d'anticorps monoclonaux contre le cytomégalovirus humain. C R Seances Acad Sci III. 1983;297(6):305–308. [PubMed] [Google Scholar]
- Middeldorp J. M., Jongsma J., The T. H. Immunofluorescence for detection of antibodies against human cytomegalovirus-induced membrane antigens. J Clin Microbiol. 1986 Sep;24(3):405–413. doi: 10.1128/jcm.24.3.405-413.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perussia B., Trinchieri G., Jackson A., Warner N. L., Faust J., Rumpold H., Kraft D., Lanier L. L. The Fc receptor for IgG on human natural killer cells: phenotypic, functional, and comparative studies with monoclonal antibodies. J Immunol. 1984 Jul;133(1):180–189. [PubMed] [Google Scholar]
- Perussia B., Trinchieri G., Lebman D., Jankiewicz J., Lange B., Rovera G. Monoclonal antibodies that detect differentiation surface antigens on human myelomonocytic cells. Blood. 1982 Feb;59(2):382–392. [PubMed] [Google Scholar]
- Plotkin S. A., Furukawa T., Zygraich N., Huygelen C. Candidate cytomegalovirus strain for human vaccination. Infect Immun. 1975 Sep;12(3):521–527. doi: 10.1128/iai.12.3.521-527.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook A. H., Smith W. J., Burdick J. F., Manischewitz J. F., Frederick W., Siegel J. P., Williams G. M., Quinnan G. V. Virus-specific cytotoxic lymphocyte responses are predictive of the outcome of cytomegalovirus infection of renal transplant recipients. Transplant Proc. 1984 Dec;16(6):1466–1469. [PubMed] [Google Scholar]
- Schrier R. D., Oldstone M. B. Recent clinical isolates of cytomegalovirus suppress human cytomegalovirus-specific human leukocyte antigen-restricted cytotoxic T-lymphocyte activity. J Virol. 1986 Jul;59(1):127–131. doi: 10.1128/jvi.59.1.127-131.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr S. E. Cytomegalovirus. Pediatr Clin North Am. 1979 May;26(2):283–293. doi: 10.1016/s0031-3955(16)33705-1. [DOI] [PubMed] [Google Scholar]
- Starr S. E., Dalton B., Garrabrant T., Paucker K., Plotkin S. A. Lymphocyte blastogenesis and interferon production in adult human leukocyte cultures stimulated with cytomegalovirus antigens. Infect Immun. 1980 Oct;30(1):17–22. doi: 10.1128/iai.30.1.17-22.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr S. E., Garrabrant T. Natural killing of cytomegalovirus-infected fibroblasts by human mononuclear leucocytes. Clin Exp Immunol. 1981 Dec;46(3):484–492. [PMC free article] [PubMed] [Google Scholar]
- Starr S. E., Smiley L., Wlodaver C., Friedman H. M., Plotkin S. A., Barker C. Natural killing of cytomegalovirus-infected targets in renal transplant recipients. Transplantation. 1984 Feb;37(2):161–164. doi: 10.1097/00007890-198402000-00009. [DOI] [PubMed] [Google Scholar]
- Waner J. L., Nierenberg J. A. Natural killing (NK) of cytomegalovirus (CMV)-infected fibroblasts: a comparison between two strains of CMV, uninfected fibroblasts, and K562 cells. J Med Virol. 1985 Jul;16(3):233–244. doi: 10.1002/jmv.1890160304. [DOI] [PubMed] [Google Scholar]


