Abstract
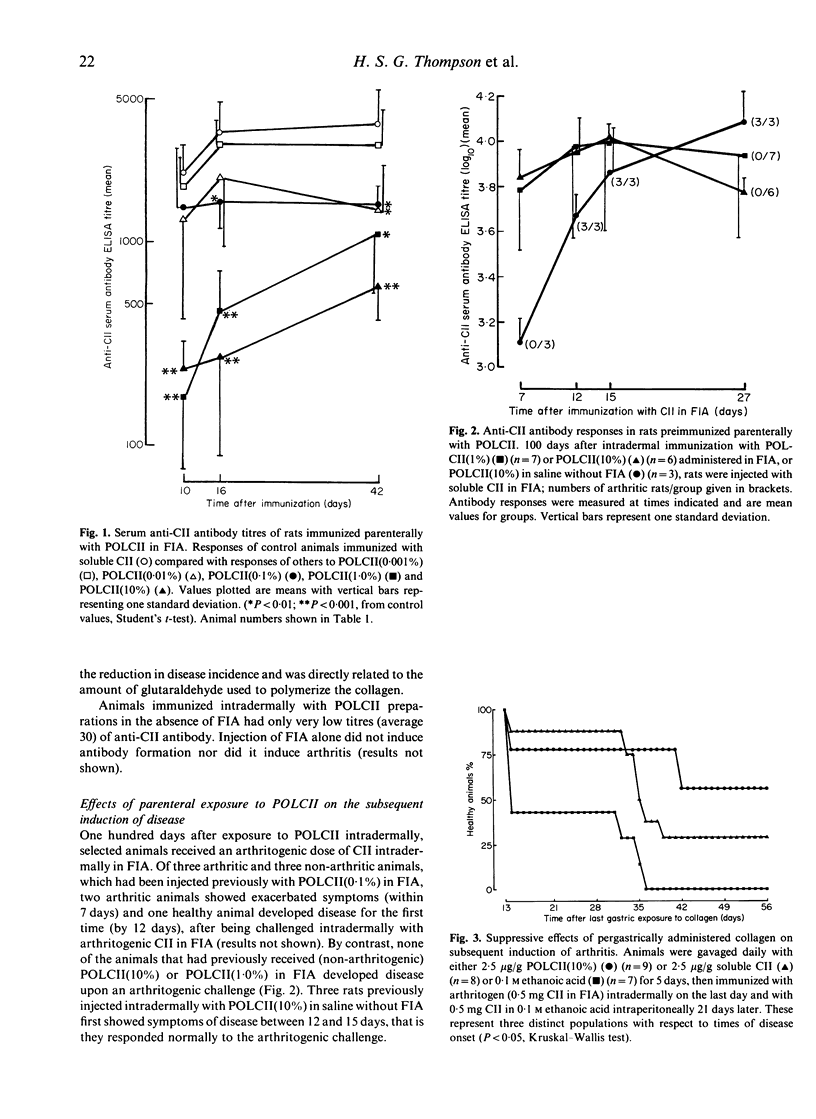
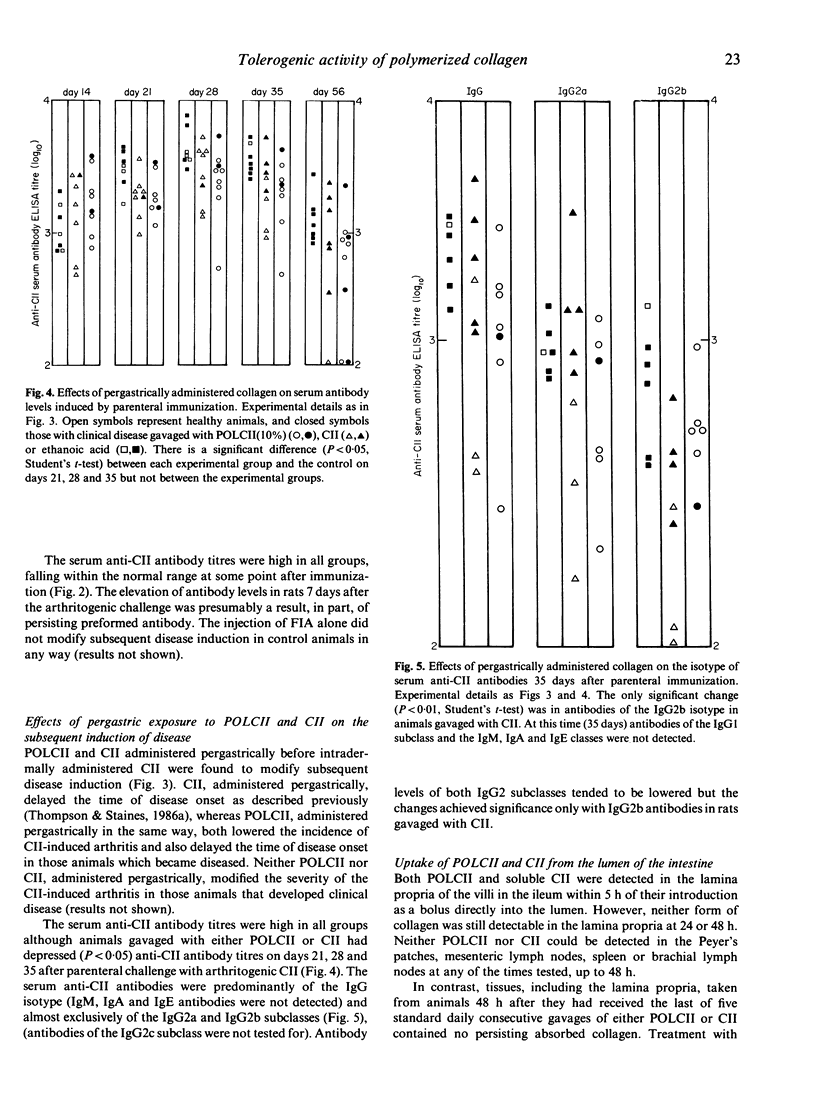
Rats were exposed parenterally or pergastrically to polymerized type II collagen (POLCII) and became resistant to the subsequent induction of disease with arthritogenic type II collagen (CII) administered intradermally in Freund's incomplete adjuvant (FIA). POLCII was prepared by cross-linking native soluble arthritogenic CII, from bovine nasal septal cartilage, with glutaraldehyde. POLCII injected intradermally in FIA did not induce arthritis. Animals treated in this manner were resistant for a period of at least 100 days to induced disease. The change in the properties of the CII from an arthritogen to a tolerogen was related to the amount of glutaraldehyde (used to polymerize the CII) which was assumed to control the extent of cross-linking of the CII. Highly cross-linked POLCII administered pergastrically, like soluble CII, was not arthritogenic but was tolerogenic, inducing a state of unresponsiveness to a challenge with arthritogenic CII. In general serum anti-CII antibody levels were higher in arthritic than in tolerized non-arthritic rats. It is concluded that the breaking of self-tolerance to CII depends upon its physical state. When polymerized and insoluble, a form analogous to that in which it exists naturally, it is tolerogenic.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brahn E., Trentham D. E. Attenuation of collagen arthritis and modulation of delayed-type hypersensitivity by type II collagen reactive T-cell lines. Cell Immunol. 1987 Oct 1;109(1):139–147. doi: 10.1016/0008-8749(87)90299-1. [DOI] [PubMed] [Google Scholar]
- Burrai I., Henderson B., Knight S. C., Staines N. A. Suppression of collagen type II-induced arthritis by transfer of lymphoid cells from rats immunized with collagen. Clin Exp Immunol. 1985 Aug;61(2):368–372. [PMC free article] [PubMed] [Google Scholar]
- Cathcart E. S., Hayes K. C., Gonnerman W. A., Lazzari A. A., Franzblau C. Experimental arthritis in a nonhuman primate. I. Induction by bovine type II collagen. Lab Invest. 1986 Jan;54(1):26–31. [PubMed] [Google Scholar]
- Challacombe S. J., Tomasi T. B., Jr Systemic tolerance and secretory immunity after oral immunization. J Exp Med. 1980 Dec 1;152(6):1459–1472. doi: 10.1084/jem.152.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiller J. M., Habicht G. S., Weigle W. O. Kinetic differences in unresponsiveness of thymus and bone marrow cells. Science. 1971 Feb 26;171(3973):813–815. doi: 10.1126/science.171.3973.813. [DOI] [PubMed] [Google Scholar]
- Courtenay J. S., Dallman M. J., Dayan A. D., Martin A., Mosedale B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature. 1980 Feb 14;283(5748):666–668. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- Cox D. S., Taubman M. A. Oral induction of the secretory antibody response by soluble and particulate antigens. Int Arch Allergy Appl Immunol. 1984;75(2):126–131. doi: 10.1159/000233602. [DOI] [PubMed] [Google Scholar]
- Elves M. W. A study of the transplantation antigens on chondrocytes from articular cartilage. J Bone Joint Surg Br. 1974 Feb;56(1):178–185. [PubMed] [Google Scholar]
- Englert M. E., Landes M. J., Oronsky A. L., Kerwar S. S. Suppression of type II collagen-induced arthritis by the intravenous administration of type II collagen or its constituent peptide alpha 1 (II) CB10. Cell Immunol. 1984 Sep;87(2):357–365. doi: 10.1016/0008-8749(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Fuchs S., Mozes E., Maoz A., Sela M. Thymus independence of a collagen-like synthetic polypeptide and of collagen, and the need for thymus and bone marrow-cell cooperation in the immune response to gelatin. J Exp Med. 1974 Jan 1;139(1):148–158. doi: 10.1084/jem.139.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman J. W., Sercarz E. E. The complexity of structures involved in T-cell activation. Annu Rev Immunol. 1983;1:465–498. doi: 10.1146/annurev.iy.01.040183.002341. [DOI] [PubMed] [Google Scholar]
- Helfgott S. M., Dynesius-Trentham R., Brahn E., Trentham D. E. An arthritogenic lymphokine in the rat. J Exp Med. 1985 Nov 1;162(5):1531–1545. doi: 10.1084/jem.162.5.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson B., Staines N. A., Burrai I., Cox J. H. The anti-arthritic and immunosuppressive effects of cyclosporine on arthritis induced in the rat by type II collagen. Clin Exp Immunol. 1984 Jul;57(1):51–56. [PMC free article] [PubMed] [Google Scholar]
- Holmdahl R., Klareskog L., Rubin K., Larsson E., Wigzell H. T lymphocytes in collagen II-induced arthritis in mice. Characterization of arthritogenic collagen II-specific T-cell lines and clones. Scand J Immunol. 1985 Sep;22(3):295–306. doi: 10.1111/j.1365-3083.1985.tb01884.x. [DOI] [PubMed] [Google Scholar]
- Holmdahl R., Rubin K., Klareskog L., Larsson E., Wigzell H. Characterization of the antibody response in mice with type II collagen-induced arthritis, using monoclonal anti-type II collagen antibodies. Arthritis Rheum. 1986 Mar;29(3):400–410. doi: 10.1002/art.1780290314. [DOI] [PubMed] [Google Scholar]
- Hom J. T., Stuart J. M., Tovey J., Chiller J. M. Murine T cells reactive to type II collagen. II. Functional characterization. J Immunol. 1986 Feb 1;136(3):776–782. [PubMed] [Google Scholar]
- Meek K. M., Chapman J. A. Glutaraldehyde-induced changes in the axially projected fine structure of collagen fibrils. J Mol Biol. 1985 Sep 20;185(2):359–370. doi: 10.1016/0022-2836(85)90409-7. [DOI] [PubMed] [Google Scholar]
- Nagler-Anderson C., Bober L. A., Robinson M. E., Siskind G. W., Thorbecke G. J. Suppression of type II collagen-induced arthritis by intragastric administration of soluble type II collagen. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7443–7446. doi: 10.1073/pnas.83.19.7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. E., Peppard J. V., Hobbs S. M. Coccidiosis: characterization of antibody responses to infection with Eimeria nieschulzi. Parasite Immunol. 1984 Jan;6(1):1–12. doi: 10.1111/j.1365-3024.1984.tb00777.x. [DOI] [PubMed] [Google Scholar]
- Rosenwasser L. J., Bhatnagar R. S., Stobo J. D. Genetic control of the murine T lymphocyte proliferative response to collagen: analysis of the molecular and cellular contributions to immunogenicity. J Immunol. 1980 Jun;124(6):2854–2859. [PubMed] [Google Scholar]
- Schoen R. T., Greene M. I., Trentham D. E. Antigen-specific suppression of type II collagen-induced arthritis by collagen-coupled spleen cells. J Immunol. 1982 Feb;128(2):717–719. [PubMed] [Google Scholar]
- Staines N. A., Hardingham T., Smith M., Henderson B. Collagen-induced arthritis in the rat: modification of immune and arthritic responses by free collagen and immune anti-collagen antiserum. Immunology. 1981 Dec;44(4):737–744. [PMC free article] [PubMed] [Google Scholar]
- Stuart J. M., Cremer M. A., Townes A. S., Kang A. H. Type II collagen-induced arthritis in rats. Passive transfer with serum and evidence that IgG anticollagen antibodies can cause arthritis. J Exp Med. 1982 Jan 1;155(1):1–16. doi: 10.1084/jem.155.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson H. S., Staines N. A. Gastric administration of type II collagen delays the onset and severity of collagen-induced arthritis in rats. Clin Exp Immunol. 1986 Jun;64(3):581–586. [PMC free article] [PubMed] [Google Scholar]
- Thompson H. S., Staines N. A. Suppression of collagen-induced arthritis with pergastrically or intravenously administered type II collagen. Agents Actions. 1986 Dec;19(5-6):318–319. doi: 10.1007/BF01971237. [DOI] [PubMed] [Google Scholar]
- Trentham D. E., Townes A. S., Kang A. H. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977 Sep 1;146(3):857–868. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman I. L. Studies on the mechanism of split tolerance in mice. Transplantation. 1966 Sep;4(5):565–571. doi: 10.1097/00007890-196609000-00002. [DOI] [PubMed] [Google Scholar]


