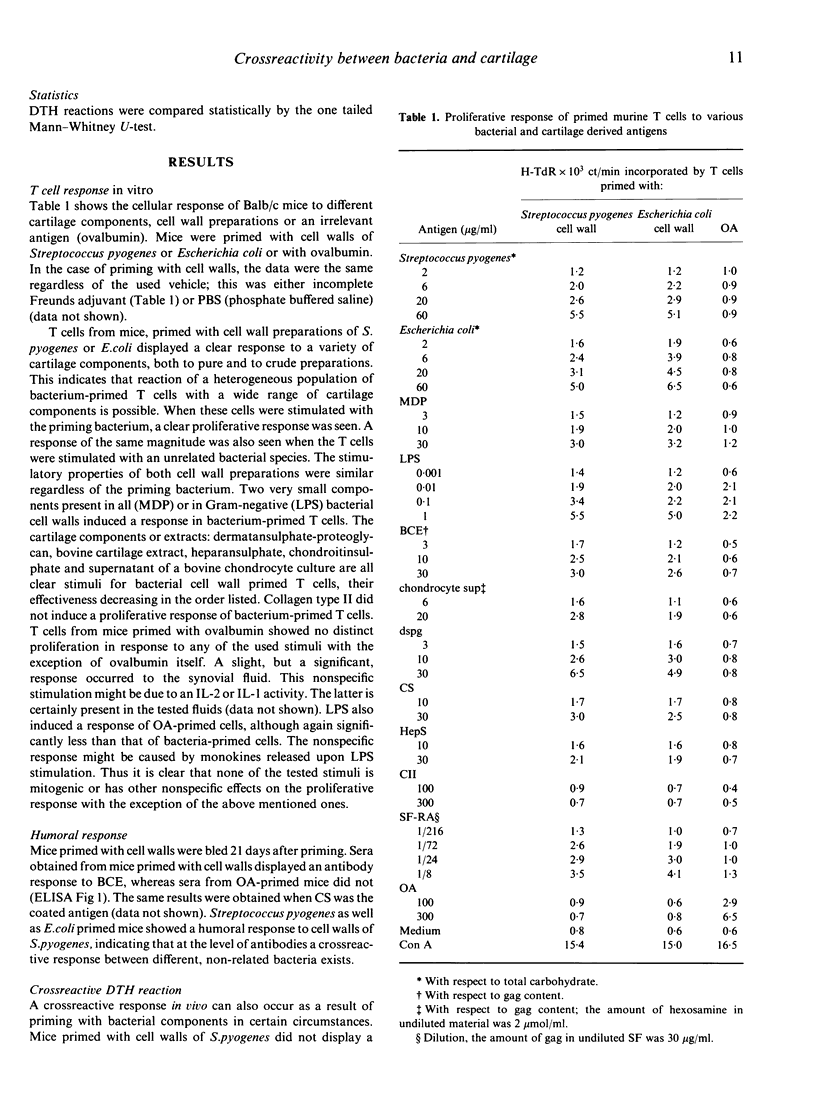
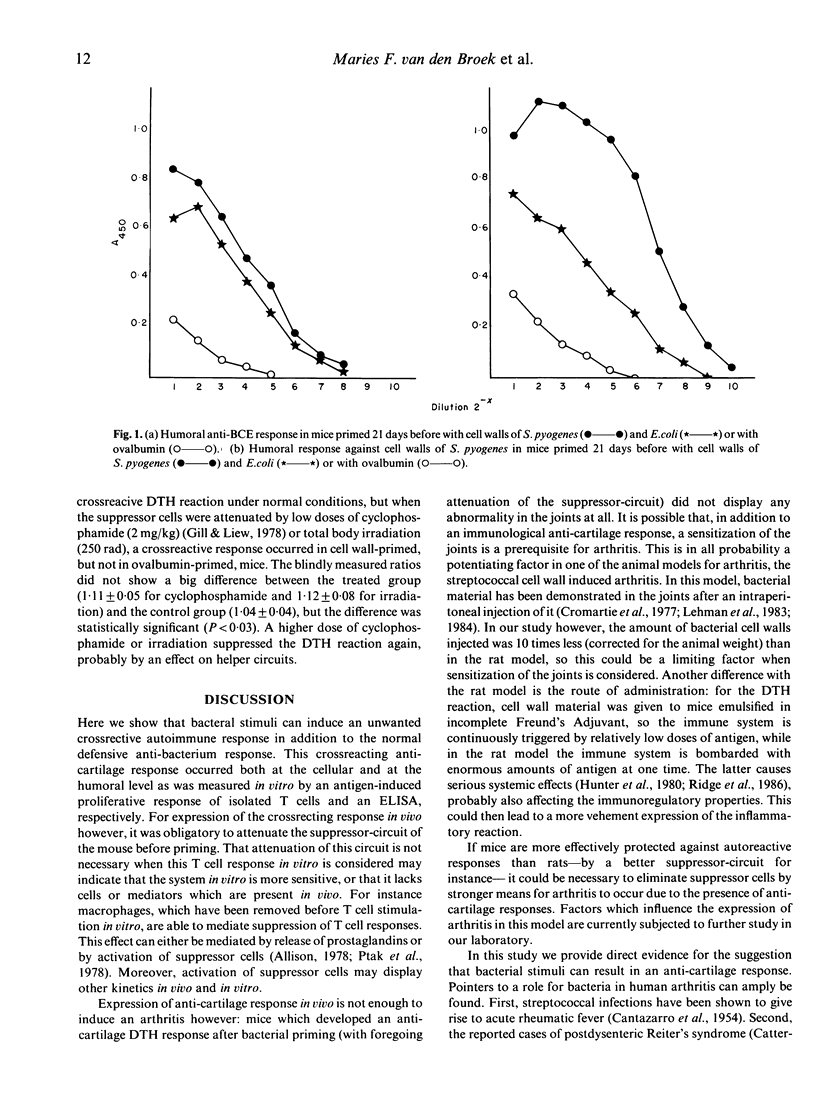
Abstract
Although different models for rheumatoid arthritis have been studied, the pathogenesis in humans remains unknown. A possible mechanism is the crossreactivity between bacterial components and the target-tissue, the cartilage. The existence of this crossreactivity is supported by various data from clinical and experimental studies. Here we provide direct evidence that priming in vivo with cell wall fragments of Streptococcus pyogenes or Escherichia coli can induce a cellular and humoral anti-cartilage response in Balb/c mice in vitro. T cells isolated from these mice can be stimulated in vitro to proliferate by a variety of antigens among which are the priming bacterium, an unrelated bacterium, small bacterial components and diverse antigens of cartilagenous origin. In bacterium-primed mice antibodies were also detected that displayed a reactivity to cartilage extract besides the reactivity to bacteria. A crossreactive response occurred in vivo in certain circumstances: a delayed type hypersensitivity reaction could be elicited in cell-wall-primed mice by challenge with cartilage extract. For the expression of this crossreactive response in vivo however, it was obligatory to attenuate the mouse's suppressor-circuit. In this paper we would suggest a mechanism for the pathology of chronic arthritis, based on repeated challenges with different bacterial stimuli.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Mechanisms by which activated macrophages inhibit lymphocyte responses. Immunol Rev. 1978;40:3–27. doi: 10.1111/j.1600-065x.1978.tb00399.x. [DOI] [PubMed] [Google Scholar]
- Burgos-Vargas R., Howard A., Ansell B. M. Antibodies to peptidoglycan in juvenile onset ankylosing spondylitis and pauciarticular onset juvenile arthritis associated with chronic iridocyclitis. J Rheumatol. 1986 Aug;13(4):760–762. [PubMed] [Google Scholar]
- CATANZARO F. J., STETSON C. A., MORRIS A. J., CHAMOVITZ R., RAMMELKAMP C. H., Jr, STOLZER B. L., PERRY W. D. The role of the streptococcus in the pathogenesis of rheumatic fever. Am J Med. 1954 Dec;17(6):749–756. doi: 10.1016/0002-9343(54)90219-3. [DOI] [PubMed] [Google Scholar]
- Cromartie W. J., Craddock J. G., Schwab J. H., Anderle S. K., Yang C. H. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med. 1977 Dec 1;146(6):1585–1602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farndale R. W., Sayers C. A., Barrett A. J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- Fillit H., Damle S. P., Gregory J. D., Volin C., Poon-King T., Zabriskie J. Sera from patients with poststreptococcal glomerulonephritis contain antibodies to glomerular heparan sulfate proteoglycan. J Exp Med. 1985 Feb 1;161(2):277–289. doi: 10.1084/jem.161.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill H. K., Liew F. Y. Regulation of delayed-type hypersensitivity. III. Effect of cyclophosphamide on the suppressor cells for delayed-type hypersensitivity to sheep erythrocytes in mice. Eur J Immunol. 1978 Mar;8(3):172–176. doi: 10.1002/eji.1830080306. [DOI] [PubMed] [Google Scholar]
- Hanglow A. C., Welsh C. J., Conn P., Pitts J. M., Rampling A., Coombs R. R. Experimental induction of rheumatoid factor and joint lesions in rabbits after intravenous injections of killed bacteria. Ann Rheum Dis. 1986 Jan;45(1):50–59. doi: 10.1136/ard.45.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holoshitz J., Klajman A., Drucker I., Lapidot Z., Yaretzky A., Frenkel A., van Eden W., Cohen I. R. T lymphocytes of rheumatoid arthritis patients show augmented reactivity to a fraction of mycobacteria cross-reactive with cartilage. Lancet. 1986 Aug 9;2(8502):305–309. doi: 10.1016/s0140-6736(86)90003-6. [DOI] [PubMed] [Google Scholar]
- Holoshitz J., Matitiau A., Cohen I. R. Arthritis induced in rats by cloned T lymphocytes responsive to mycobacteria but not to collagen type II. J Clin Invest. 1984 Jan;73(1):211–215. doi: 10.1172/JCI111193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holoshitz J., Naparstek Y., Ben-Nun A., Cohen I. R. Lines of T lymphocytes induce or vaccinate against autoimmune arthritis. Science. 1983 Jan 7;219(4580):56–58. doi: 10.1126/science.6336851. [DOI] [PubMed] [Google Scholar]
- Hunter N., Anderle S. K., Brown R. R., Dalldorf F. G., Clark R. L., Cromartie W. J., Schwab J. H. Cell-mediated immune response during experimental arthritis induced in rats with streptococcal cell walls. Clin Exp Immunol. 1980 Dec;42(3):441–449. [PMC free article] [PubMed] [Google Scholar]
- Johnson P. M., Phua K. K., Perkins H. R., Hart C. A., Bucknall R. C. Antibody to streptococcal cell wall peptidoglycan-polysaccharide polymers in seropositive and seronegative rheumatic disease. Clin Exp Immunol. 1984 Jan;55(1):115–124. [PMC free article] [PubMed] [Google Scholar]
- Koga T., Kakimoto K., Hirofuji T., Kotani S., Ohkuni H., Watanabe K., Okada N., Okada H., Sumiyoshi A., Saisho K. Acute joint inflammation in mice after systemic injection of the cell wall, its peptidoglycan, and chemically defined peptidoglycan subunits from various bacteria. Infect Immun. 1985 Oct;50(1):27–34. doi: 10.1128/iai.50.1.27-34.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman T. J., Allen J. B., Plotz P. H., Wilder R. L. Lactobacillus casei cell wall-induced arthritis in rats: cell wall fragment distribution and persistence in chronic arthritis-susceptible LEW/N and -resistant F344/N rats. Arthritis Rheum. 1984 Aug;27(8):939–942. doi: 10.1002/art.1780270815. [DOI] [PubMed] [Google Scholar]
- Lehman T. J., Allen J. B., Plotz P. H., Wilder R. L. Polyarthritis in rats following the systemic injection of Lactobacillus casei cell walls in aqueous suspension. Arthritis Rheum. 1983 Oct;26(10):1259–1265. doi: 10.1002/art.1780261013. [DOI] [PubMed] [Google Scholar]
- PEARSON C. M., WAKSMAN B. H., SHARP J. T. Studies of arthritis and other lesions induced in rats by injection of mycobacterial adjuvant. V. Changes affecting the skin and mucous membranes. Comparison of the experimental process with human disease. J Exp Med. 1961 Mar 1;113:485–510. doi: 10.1084/jem.113.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEARSON C. M., WOOD F. D. PASSIVE TRANSFER OF ADJUVANT ARTHRITIS BY LYMPH NODE OR SPLEEN CELLS. J Exp Med. 1964 Oct 1;120:547–560. doi: 10.1084/jem.120.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo I., Carafa C., Dziarski R., Levinson A. I. Analysis of in vitro polyclonal B cell differentiation responses to bacterial peptidoglycan and pokeweed mitogen in rheumatoid arthritis. Clin Exp Immunol. 1984 May;56(2):253–262. [PMC free article] [PubMed] [Google Scholar]
- Ptak W., Zembala M., Gershon R. K. Intermediary role of macrophages in the passage of suppressor signals between T-cell subsets. J Exp Med. 1978 Aug 1;148(2):424–434. doi: 10.1084/jem.148.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge S. C., Zabriske J. B., Oronsky A. L., Kerwar S. S. Streptococcal cell wall arthritis: studies with nude (athymic) inbred Lewis rats. Cell Immunol. 1985 Nov;96(1):231–234. doi: 10.1016/0008-8749(85)90354-5. [DOI] [PubMed] [Google Scholar]
- Ridge S. C., Zabriskie J. B., Osawa H., Diamantstein T., Oronsky A. L., Kerwar S. S. Administration of group A streptococcal cell walls to rats induces an interleukin 2 deficiency. J Exp Med. 1986 Jul 1;164(1):327–332. doi: 10.1084/jem.164.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., Gilkerson E. Quantitation of glycosaminoglycan hexosamine using 3-methyl-2-benzothiazolone hydrazone hydrochloride. Anal Biochem. 1979 Oct 1;98(2):478–480. doi: 10.1016/0003-2697(79)90170-2. [DOI] [PubMed] [Google Scholar]
- van Eden W., Holoshitz J., Nevo Z., Frenkel A., Klajman A., Cohen I. R. Arthritis induced by a T-lymphocyte clone that responds to Mycobacterium tuberculosis and to cartilage proteoglycans. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5117–5120. doi: 10.1073/pnas.82.15.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]


