Abstract
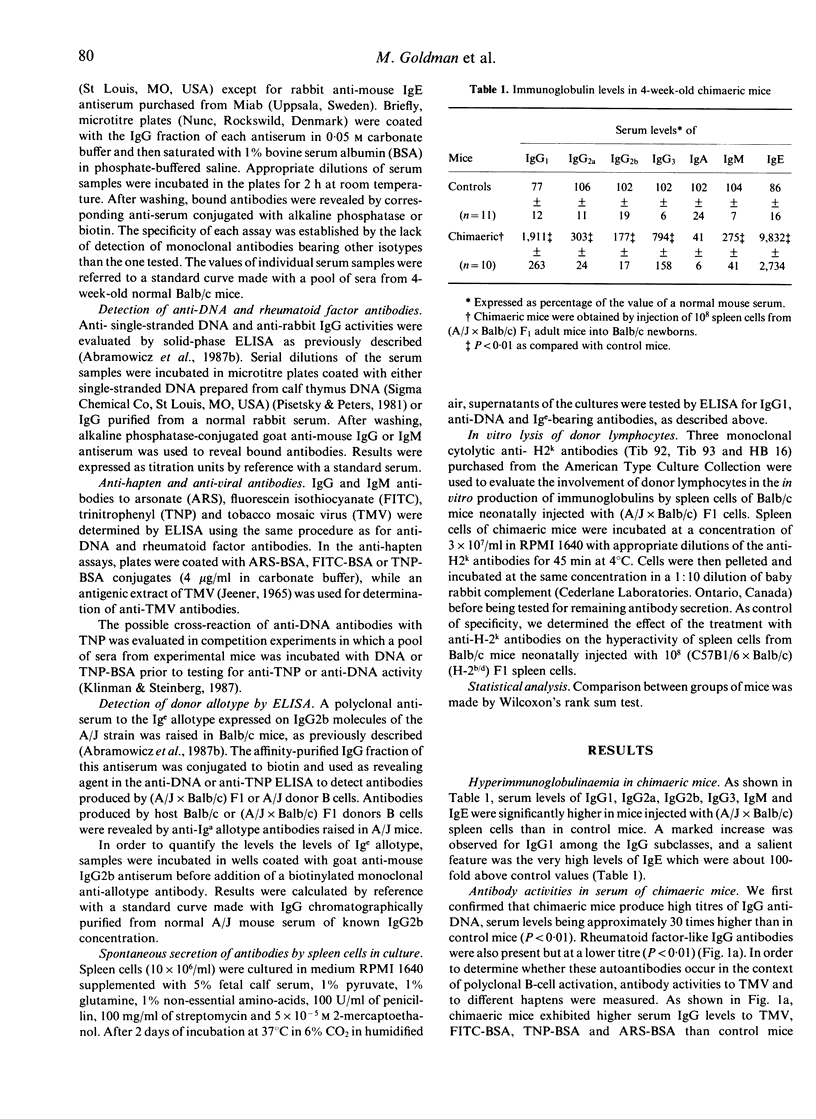
Balb/c mice made chimaeric by neonatal injection of semi-allogeneic (A/J x Balb/c)F1 hybrid spleen cells develop anti-DNA and rheumatoid factor-like antibodies in the context of hypergammaglobulinaemia with marked elevation of IgG1 and IgE serum levels. Chimaeric mice also display increased levels of antibodies to different haptens and to tobacco mosaic virus (TMV). The allotypic marker of the A/J strain is present on anti-DNA and anti-hapten antibodies. In addition, spleen cells of chimaeric mice spontaneously produce high levels of IgG1 and anti-DNA antibodies in vitro and this hyperactivity is abolished after lysis of donor lymphocytes. These findings indicate that polyclonal activation of donor B cells plays an important role in this model of autoimmunity.
Full text
PDF
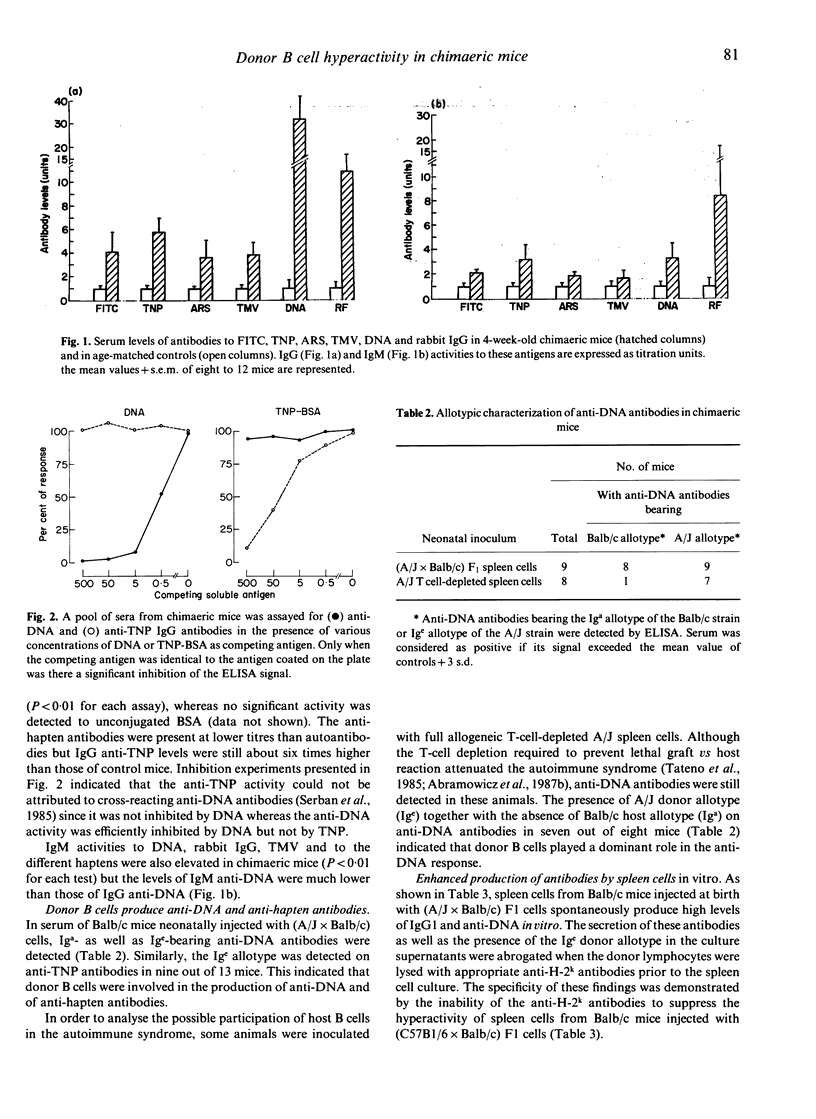

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramowicz D., Goldman M., Bruyns C., Lambert P., Thoua Y., Toussaint C. Autoimmune disease after neonatal injection of semi-allogeneic spleen cells in mice: involvement of donor B and T cells and characterization of glomerular deposits. Clin Exp Immunol. 1987 Oct;70(1):61–67. [PMC free article] [PubMed] [Google Scholar]
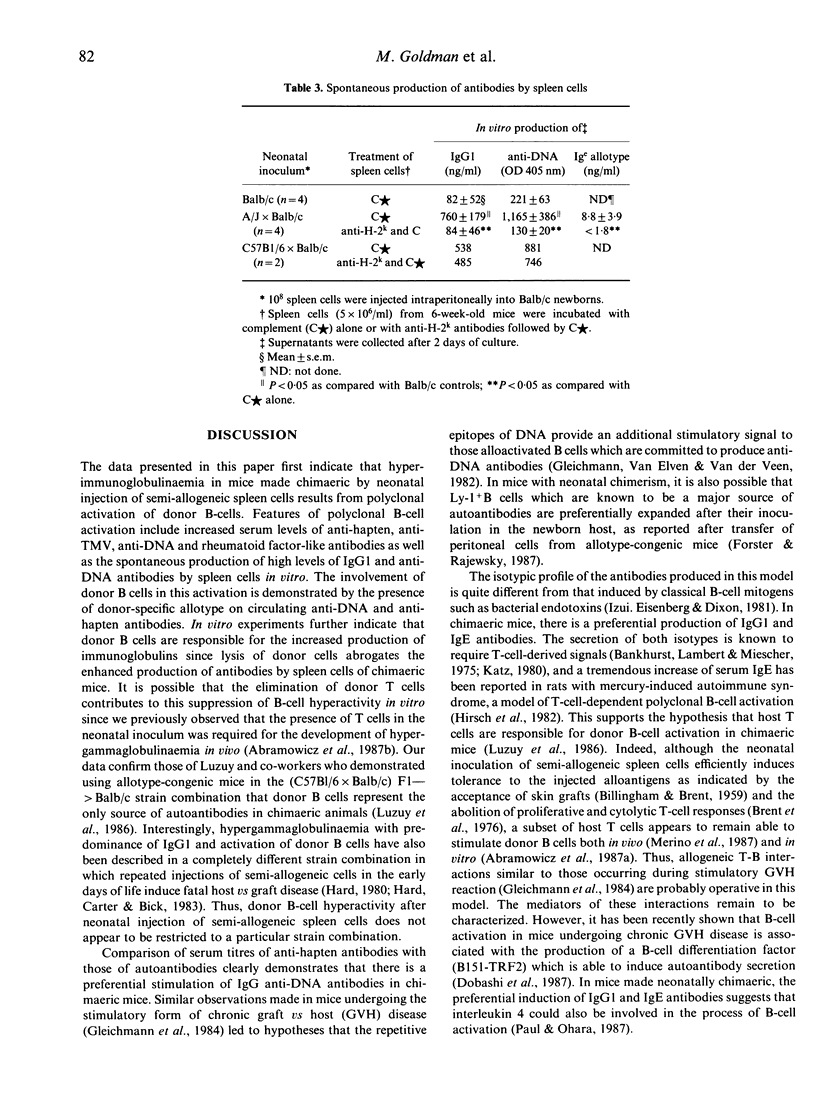
- Bankhurst A. D., Lambert P. H., Miescher P. A. Studies on the thymic dependence of the immunoglobulin classes of the mouse (38570). Proc Soc Exp Biol Med. 1975 Feb;148(2):501–504. doi: 10.3181/00379727-148-38570. [DOI] [PubMed] [Google Scholar]
- Brent L., Brooks C. G., Medawar P. B., Simpson E. Transplantation tolerance. Br Med Bull. 1976 May;32(2):101–106. doi: 10.1093/oxfordjournals.bmb.a071339. [DOI] [PubMed] [Google Scholar]
- Dobashi K., Ono S., Murakami S., Takahama Y., Katoh Y., Hamaoka T. Polyclonal B cell activation by a B cell differentiation factor, B151-TRF2. III. B151-TRF2 as a B cell differentiation factor closely associated with autoimmune disease. J Immunol. 1987 Feb 1;138(3):780–787. [PubMed] [Google Scholar]
- Förster I., Rajewsky K. Expansion and functional activity of Ly-1+ B cells upon transfer of peritoneal cells into allotype-congenic, newborn mice. Eur J Immunol. 1987 Apr;17(4):521–528. doi: 10.1002/eji.1830170414. [DOI] [PubMed] [Google Scholar]
- Gleichmann E., Van Elven E. H., Van der Veen J. P. A systemic lupus erythematosus (SLE)-like disease in mice induced by abnormal T-B cell cooperation. Preferential formation of autoantibodies characteristic of SLE. Eur J Immunol. 1982 Feb;12(2):152–159. doi: 10.1002/eji.1830120210. [DOI] [PubMed] [Google Scholar]
- Goldman M., Feng H. M., Engers H., Hochman A., Louis J., Lambert P. H. Autoimmunity and immune complex disease after neonatal induction of transplantation tolerance in mice. J Immunol. 1983 Jul;131(1):251–258. [PubMed] [Google Scholar]
- Hard R. C., Jr, Carter W. H., Bick P. H. Sensitized (T6 X RFM)F1 donor B cells are engrafted and synthesize antibodies without further antigenic stimulation in RFM recipients with allogenic host-vs-graft disease. J Immunol. 1983 Dec;131(6):2623–2629. [PubMed] [Google Scholar]
- Hard R. C., Jr Sequential changes in serum immunoglobulin levels in young RFM mice with host-versus-graft disease. Immunology. 1980 Apr;39(4):453–461. [PMC free article] [PubMed] [Google Scholar]
- Hirsch F., Couderc J., Sapin C., Fournie G., Druet P. Polyclonal effect of HgCl2 in the rat, its possible role in an experimental autoimmune disease. Eur J Immunol. 1982 Jul;12(7):620–625. doi: 10.1002/eji.1830120716. [DOI] [PubMed] [Google Scholar]
- Izui S., Eisenberg R. A., Dixon F. J. Subclass-restricted IgG polyclonal antibody production in mice injected with lipid A-rich lipopolysaccharides. J Exp Med. 1981 Feb 1;153(2):324–338. doi: 10.1084/jem.153.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEENER R. EFFECTS ON THE ANTIGENIC DETERIMINANTS OF TOBACCO MOSAIC VIRUS OF A SMALL NUMBER OF BASE REPLACEMENTS IN THE RIBONUCLEIC ACID. Virology. 1965 May;26:10–15. doi: 10.1016/0042-6822(65)90020-6. [DOI] [PubMed] [Google Scholar]
- Katz D. H. Recent studies on the regulation of IgE antibody synthesis in experimental animals and man. Immunology. 1980 Sep;41(1):1–24. [PMC free article] [PubMed] [Google Scholar]
- Klinman D. M., Steinberg A. D. Systemic autoimmune disease arises from polyclonal B cell activation. J Exp Med. 1987 Jun 1;165(6):1755–1760. doi: 10.1084/jem.165.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzuy S., Merino J., Engers H., Izui S., Lambert P. H. Autoimmunity after induction of neonatal tolerance to alloantigens: role of B cell chimerism and F1 donor B cell activation. J Immunol. 1986 Jun 15;136(12):4420–4426. [PubMed] [Google Scholar]
- Paul W. E., Ohara J. B-cell stimulatory factor-1/interleukin 4. Annu Rev Immunol. 1987;5:429–459. doi: 10.1146/annurev.iy.05.040187.002241. [DOI] [PubMed] [Google Scholar]
- Pisetsky D. S., Peters D. V. A simple enzyme-linked immunosorbent assay for antibodies to native DNA. J Immunol Methods. 1981;41(2):187–200. doi: 10.1016/0022-1759(81)90242-8. [DOI] [PubMed] [Google Scholar]
- Serban D., Rordorf-Adam C., Sun Y. Z., Gordon J. Murine polyspecific antibodies. I. Monoclonal and serum anti-DNA antibodies cross-reactive with 2,4,6-trinitrophenyl derivatives. J Immunol. 1985 Nov;135(5):3122–3127. [PubMed] [Google Scholar]
- Tateno M., Kondo N., Itoh T., Yoshiki T. Autoimmune disease and malignant lymphoma associated with host-versus-graft disease in mice. Clin Exp Immunol. 1985 Dec;62(3):535–544. [PMC free article] [PubMed] [Google Scholar]


