Abstract
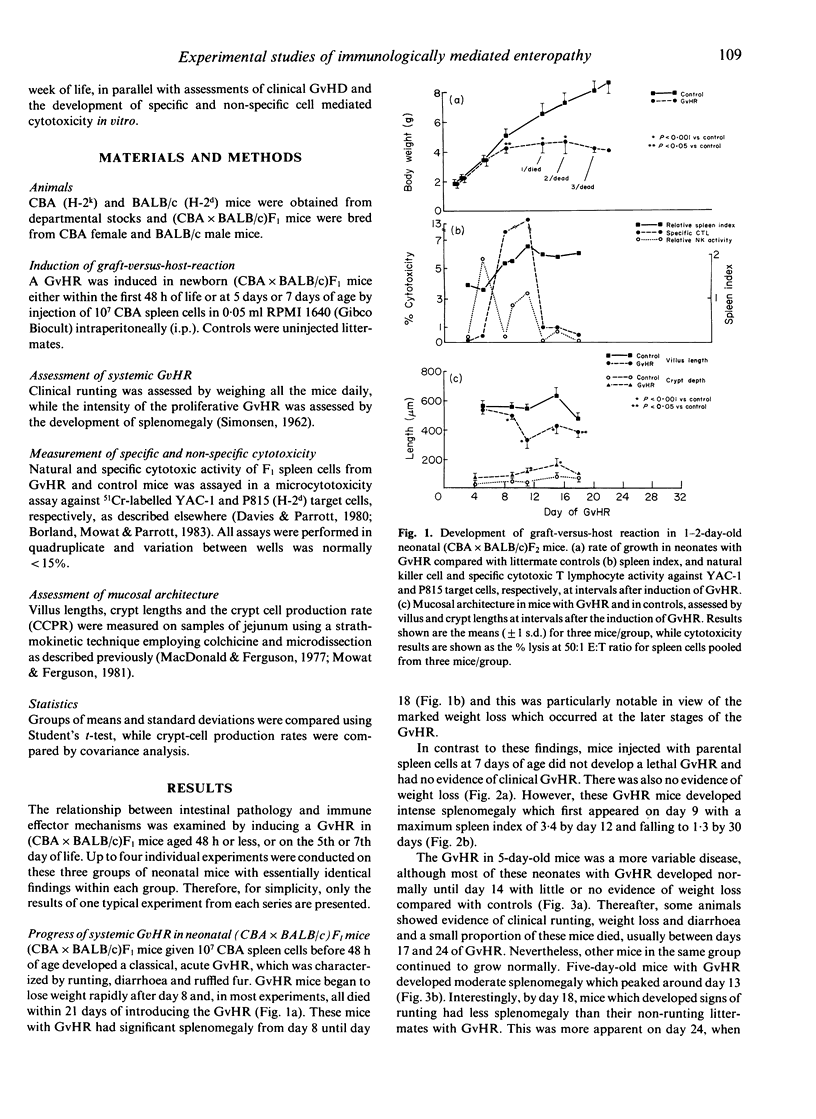
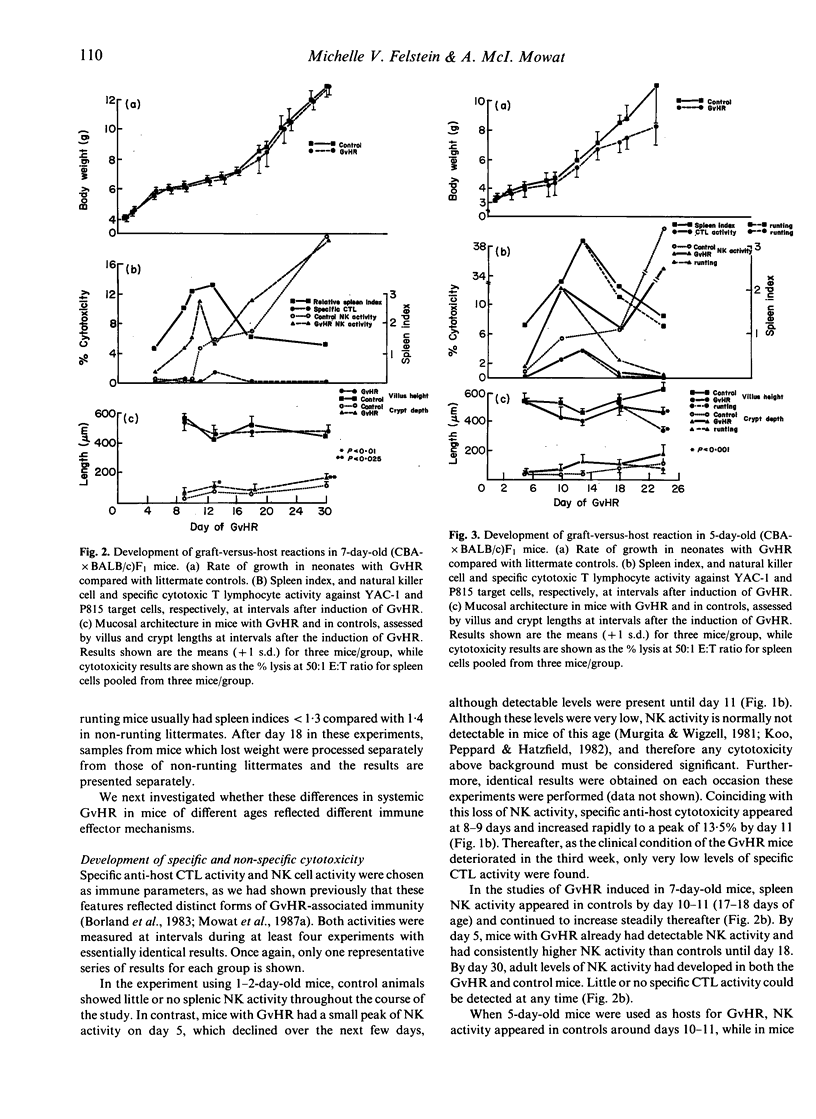
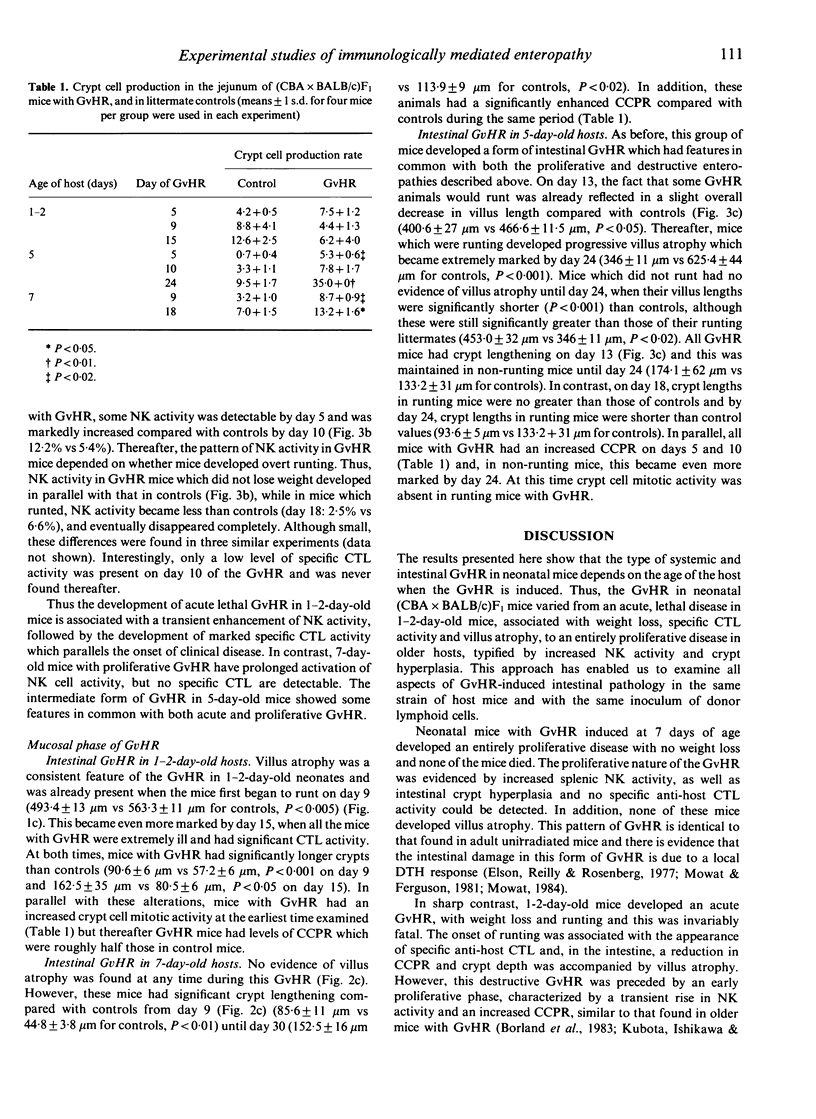
We have used the intestinal phase of the graft-versus-host-reaction (GvHR) in unirradiated F1 mice as a model for enteropathy due to cell-mediated immunity (CMI). Injection of neonatal (CBA x BALB/c)F1 mice less than 48 h old with CBA spleen cells produced an acute GvHR, which was associated with runting and severe intestinal damage, characterized by villus atrophy. These animals developed specific cytotoxic T lymphocyte (CTL) activity and invariably died. In contrast, 7-day-old F1 mice with GvHR developed a proliferative GvHR, characterized by intense splenomegaly, NK cell activation and intestinal crypt hyperplasia. These mice did not lose weight, had no villus atrophy or CTL activity and all recovered. A similar proliferative phase was also found to precede the established GvHR in 1-2-day-old hosts. Induction of a GvHR in 5-day-old hosts produced a disease with some characteristics of both proliferative and destructive GvHR, with some mice developing weight loss and villus atrophy, while others showed only crypt hyperplasia and NK cell activation. However, there was very little specific CTL activity in any of these animals. These results indicate that markedly different forms of GvHR can be induced in mice during the first week of life and that these are associated with different pathological effects. Although the immunological mechanisms which are activated may also differ between the types of GvHR, our findings support the hypothesis that intestinal damage which includes villus atrophy is merely a progressive form of the delayed type hypersensitivity responsible for a proliferative enteropathy.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borland A., Mowat A. M., Parrott D. M. Augmentation of intestinal and peripheral natural killer cell activity during the graft-versus-host reaction in mice. Transplantation. 1983 Nov;36(5):513–519. doi: 10.1097/00007890-198311000-00009. [DOI] [PubMed] [Google Scholar]
- Davies M. D., Parrott D. M. The early appearance of specific cytotoxic T cells in murine gut mucosa. Clin Exp Immunol. 1980 Nov;42(2):273–279. [PMC free article] [PubMed] [Google Scholar]
- Elson C. O., Reilly R. W., Rosenberg I. H. Small intestinal injury in the graft versus host reaction: an innocent bystander phenomenon. Gastroenterology. 1977 May;72(5 Pt 1):886–889. [PubMed] [Google Scholar]
- Ferguson A., Jarrett E. E. Hypersensitivity reactions in small intestine. I Thymus dependence of experimental 'partial villous atrophy'. Gut. 1975 Feb;16(2):114–117. doi: 10.1136/gut.16.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy-Grand D., Vassalli P. Gut injury in mouse graft-versus-host reaction. Study of its occurrence and mechanisms. J Clin Invest. 1986 May;77(5):1584–1595. doi: 10.1172/JCI112474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo G. C., Peppard J. R., Hatzfeld A. Ontogeny of Nk-1+ natural killer cells. I. Promotion of Nk-1+ cells in fetal, baby, and old mice. J Immunol. 1982 Aug;129(2):867–871. [PubMed] [Google Scholar]
- Kubota E., Ishikawa H., Saito K. Modulation of F1 cytotoxic potentials by GvHR. Host- and donor-derived cytotoxic lymphocytes arise in the unirradiated F1 host spleens under the condition of GvHR-associated immunosuppression. J Immunol. 1983 Sep;131(3):1142–1148. [PubMed] [Google Scholar]
- Lund E. K., Bruce M. G., Smith M. W., Ferguson A. Selective effects of graft-versus-host reaction on disaccharidase expression by mouse jejunal enterocytes. Clin Sci (Lond) 1986 Aug;71(2):189–198. doi: 10.1042/cs0710189. [DOI] [PubMed] [Google Scholar]
- MacDonald T. T., Ferguson A. Hypersensitivity reactions in the small intestine. III. The effects of allograft rejection and of graft-versus-host disease on epithelial cell kinetics. Cell Tissue Kinet. 1977 Jul;10(4):301–312. [PubMed] [Google Scholar]
- Mowat A. M., Borland A., Parrott D. M. Augmentation of natural killer cell activity by anti-host delayed-type hypersensitivity during the graft-versus-host reaction in mice. Scand J Immunol. 1985 Oct;22(4):389–399. doi: 10.1111/j.1365-3083.1985.tb01897.x. [DOI] [PubMed] [Google Scholar]
- Mowat A. M., Felstein M. V., Baca M. E. Experimental studies of immunologically mediated enteropathy. III. Severe and progressive enteropathy during a graft-versus-host reaction in athymic mice. Immunology. 1987 Jun;61(2):185–188. [PMC free article] [PubMed] [Google Scholar]
- Mowat A. M., Felstein M. V. Experimental studies of immunologically mediated enteropathy. II. Role of natural killer cells in the intestinal phase of murine graft-versus-host reaction. Immunology. 1987 Jun;61(2):179–183. [PMC free article] [PubMed] [Google Scholar]
- Mowat A. M., Ferguson A. Intraepithelial lymphocyte count and crypt hyperplasia measure the mucosal component of the graft-versus-host reaction in mouse small intestine. Gastroenterology. 1982 Aug;83(2):417–423. [PubMed] [Google Scholar]
- Murgita R. A., Wigzell H. Regulation of immune functions in the fetus and newborn. Prog Allergy. 1981;29:54–133. [PubMed] [Google Scholar]
- REILLY R. W., KIRSNER J. B. RUNT INTESTINAL DISEASE. Lab Invest. 1965 Jan;14:102–107. [PubMed] [Google Scholar]
- SIMONSEN M. Graft versus host reactions. Their natural history, and applicability as tools of research. Prog Allergy. 1962;6:349–467. [PubMed] [Google Scholar]


