Abstract
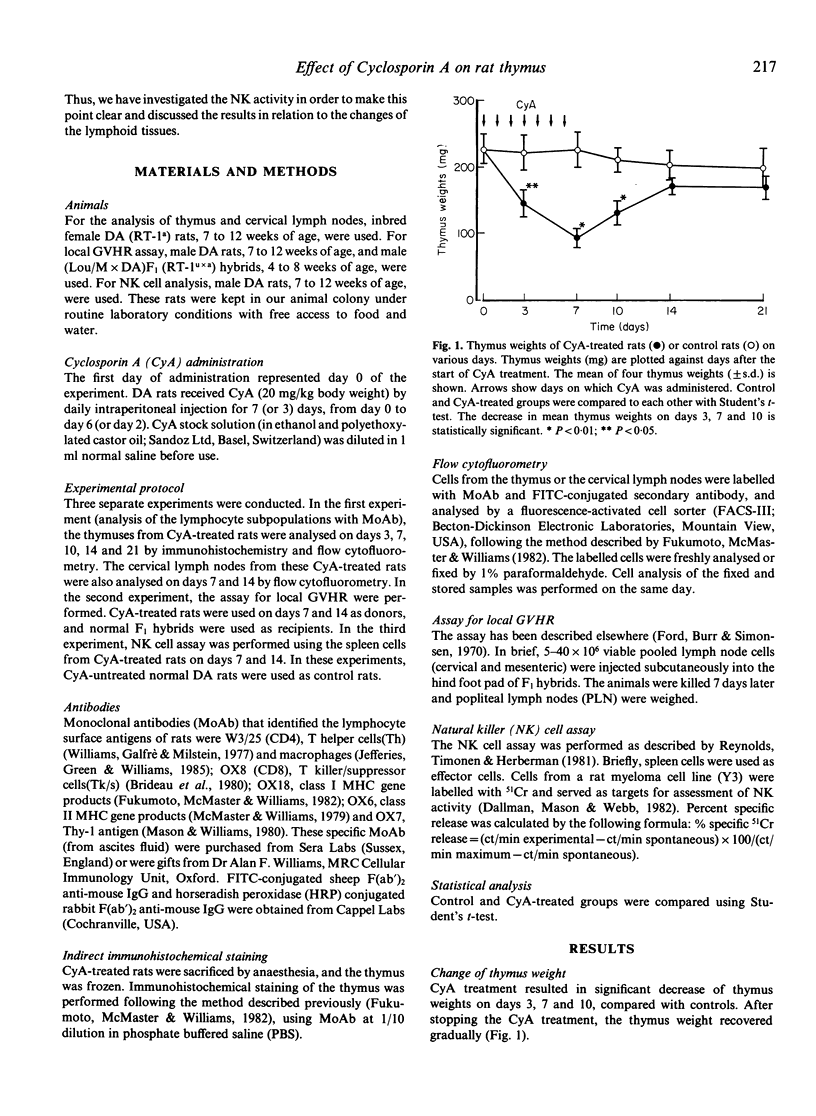
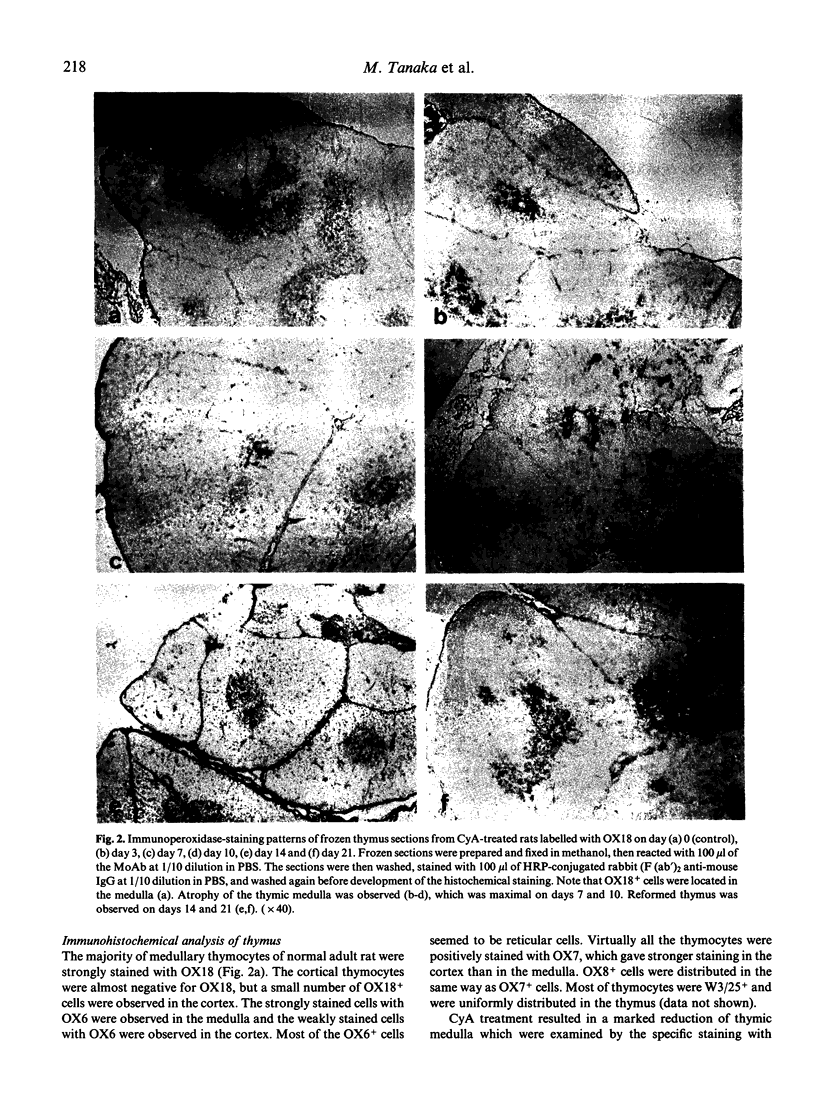
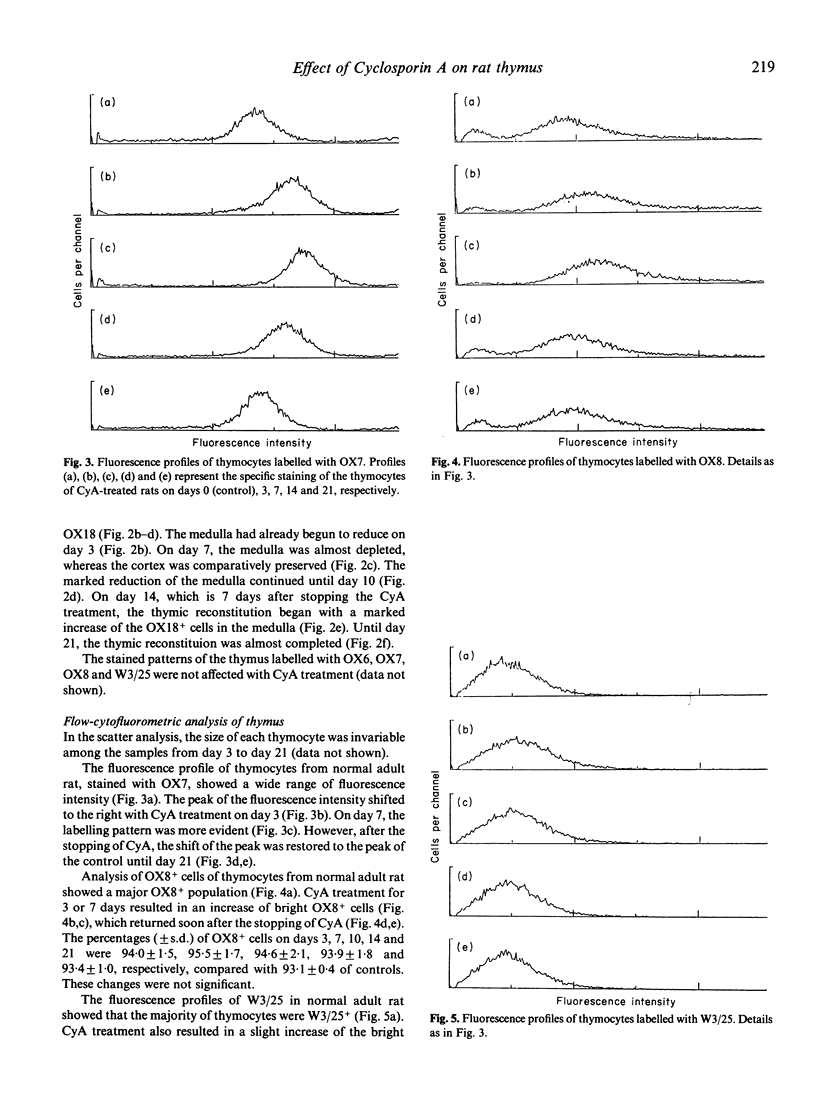
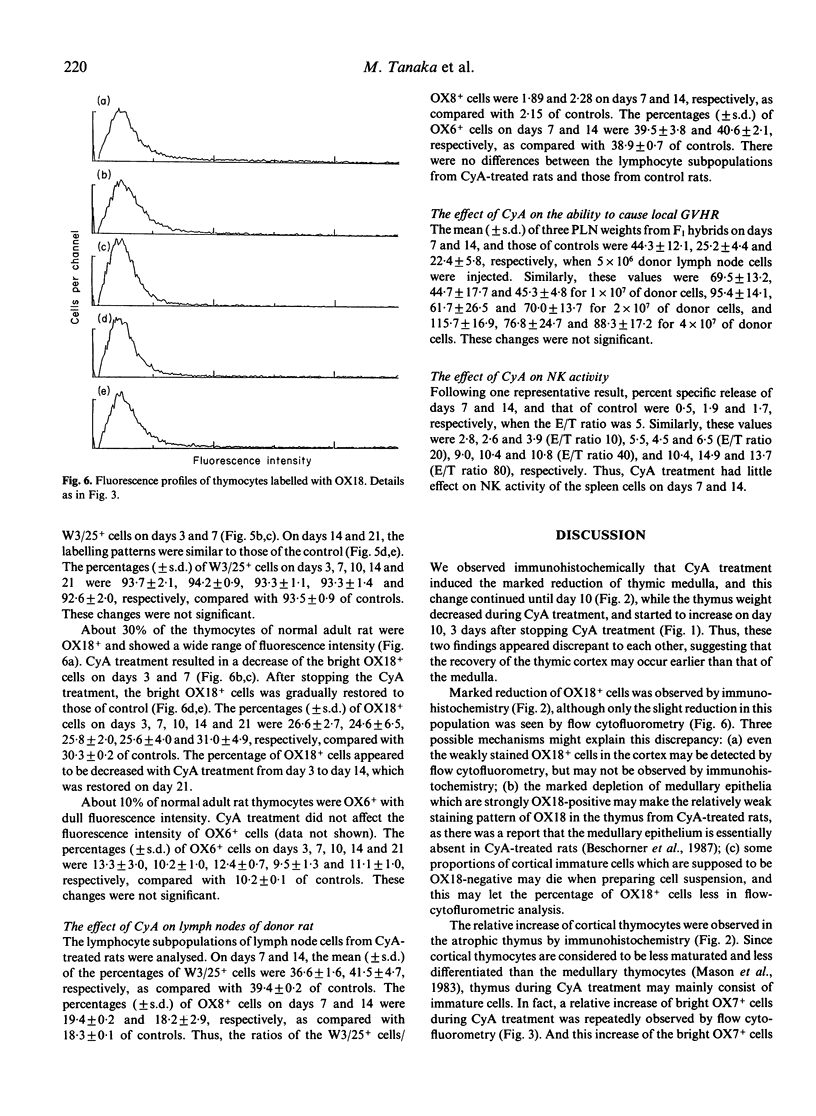
The effect of Cyclosporin A (CyA) administration 20 mg/kg body weight i.p. for 3 or 7 days on rat lymphoid tissues, especially on the thymus, and the recovery after stopping CyA treatment were investigated by both immunoperoxidase technique and flow cytofluorometry using monoclonal antibodies against rat lymphocytes: OX6, OX7, OX8, OX18 and W3/25. The marked reduction of thymic medulla with CyA treatment was clearly demonstrated by staining with OX18. This change was maximal 7 to 10 days after the start of CyA administration. The obvious restitution of the thymic medulla occurred about 7 days after stopping CyA and was almost completed within 14 days. Flow-cytofluorometric analysis of the thymus showed that the percentages of positive cells labelled with OX7, OX8, OX18 and W3/25 appeared to be not changed except for OX18 during and after CyA treatment. However, the expression of each antigen per cell changed in the amount; the peak of fluorescence intensity of OX7+ cells showed a temporary shift to the right during CyA treatment. Bright positive cell populations for each OX8 and W3/25 increased relatively during CyA treatment, and reverted to the normal levels soon after stopping the CyA treatment. On the other hand, bright OX18+ cells decreased with CyA treatment, but this change recovered gradually after stopping the CyA treatment. Treatment with CyA gave no significant changes in the flow-cytofluorometric analyses for these antibodies on lymph node cells. Natural killer cell activity and the ability to cause local graft-versus-host reaction were not inhibited with CyA treatment. These results suggest that CyA inhibits the proliferation and differentiation of thymocytes, or that CyA makes thymocytes migrate rapidly from cortex to periphery.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin W. M., 3rd, Hutchinson I. F., Meijer C. J., Tilney N. L. Immune responses to organ allografts. III. Marked decrease in medullary thymocytes and splenic T lymphocytes after cyclosporin A treatment. Transplantation. 1981 Feb;31(2):117–120. [PubMed] [Google Scholar]
- Beschorner W. E., Namnoum J. D., Hess A. D., Shinn C. A., Santos G. W. Cyclosporin A and the thymus. Immunopathology. Am J Pathol. 1987 Mar;126(3):487–496. [PMC free article] [PubMed] [Google Scholar]
- Blair J. T., Thomson A. W., Whiting P. H., Davidson R. J., Simpson J. G. Toxicity of the immune suppressant cyclosporin A in the rat. J Pathol. 1982 Oct;138(2):163–178. doi: 10.1002/path.1711380206. [DOI] [PubMed] [Google Scholar]
- Borel J. F., Feurer C., Magnée C., Stähelin H. Effects of the new anti-lymphocytic peptide cyclosporin A in animals. Immunology. 1977 Jun;32(6):1017–1025. [PMC free article] [PubMed] [Google Scholar]
- Brideau R. J., Carter P. B., McMaster W. R., Mason D. W., Williams A. F. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980 Aug;10(8):609–615. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- Cyclosporine. Experimental transplantation. Bone marrow. Transplant Proc. 1983 Dec;15(4 Suppl 1-2):3035–3049. [PubMed] [Google Scholar]
- Dallman M. J., Mason D. W., Webb M. The roles of host and donor cells in the rejection of skin allografts by T cell-deprived rats injected with syngeneic T cells. Eur J Immunol. 1982 Jun;12(6):511–518. doi: 10.1002/eji.1830120612. [DOI] [PubMed] [Google Scholar]
- Ford W. L., Burr W., Simonsen M. A lymph node weight assay for the graft-versus-host activity of rat lymphoid cells. Transplantation. 1970 Sep;10(3):258–266. doi: 10.1097/00007890-197009000-00007. [DOI] [PubMed] [Google Scholar]
- Fukumoto T., McMaster W. R., Williams A. F. Mouse monoclonal antibodies against rat major histocompatibility antigens. Two Ia antigens and expression of Ia and class I antigens in rat thymus. Eur J Immunol. 1982 Mar;12(3):237–243. doi: 10.1002/eji.1830120313. [DOI] [PubMed] [Google Scholar]
- Introna M., Allavena P., Spreafico F., Mantovani A. Inhibition of human natural killer activity by cyclosporin A. Transplantation. 1981 Feb;31(2):113–116. doi: 10.1097/00007890-198102000-00004. [DOI] [PubMed] [Google Scholar]
- Jefferies W. A., Green J. R., Williams A. F. Authentic T helper CD4 (W3/25) antigen on rat peritoneal macrophages. J Exp Med. 1985 Jul 1;162(1):117–127. doi: 10.1084/jem.162.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D. W., Williams A. F. The kinetics of antibody binding to membrane antigens in solution and at the cell surface. Biochem J. 1980 Apr 1;187(1):1–20. doi: 10.1042/bj1870001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster W. R., Williams A. F. Identification of Ia glycoproteins in rat thymus and purification from rat spleen. Eur J Immunol. 1979 Jun;9(6):426–433. doi: 10.1002/eji.1830090603. [DOI] [PubMed] [Google Scholar]
- Reynolds C. W., Timonen T., Herberman R. B. Natural killer (NK) cell activity in the rat. I. Isolation and characterization of the effector cells. J Immunol. 1981 Jul;127(1):282–287. [PubMed] [Google Scholar]
- Ryffel B., Deyssenroth H., Borel J. F. Cyclosporin A: effects on the mouse thymus. Agents Actions. 1981 Jul;11(4):373–379. doi: 10.1007/BF01982473. [DOI] [PubMed] [Google Scholar]
- Shao-Hsien C., Lang I., Gunn H., Lydyard P. Effect of in vitro cyclosporin. A treatment on human natural and antibody-dependent cell-mediated cytotoxicity. Transplantation. 1983 Feb;35(2):127–129. doi: 10.1097/00007890-198302000-00004. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Galfrè G., Milstein C. Analysis of cell surfaces by xenogeneic myeloma-hybrid antibodies: differentiation antigens of rat lymphocytes. Cell. 1977 Nov;12(3):663–673. doi: 10.1016/0092-8674(77)90266-5. [DOI] [PubMed] [Google Scholar]
- Williams A. F. Surface molecules and cell interactions. J Theor Biol. 1982 Sep 21;98(2):221–234. doi: 10.1016/0022-5193(82)90260-0. [DOI] [PubMed] [Google Scholar]