Abstract
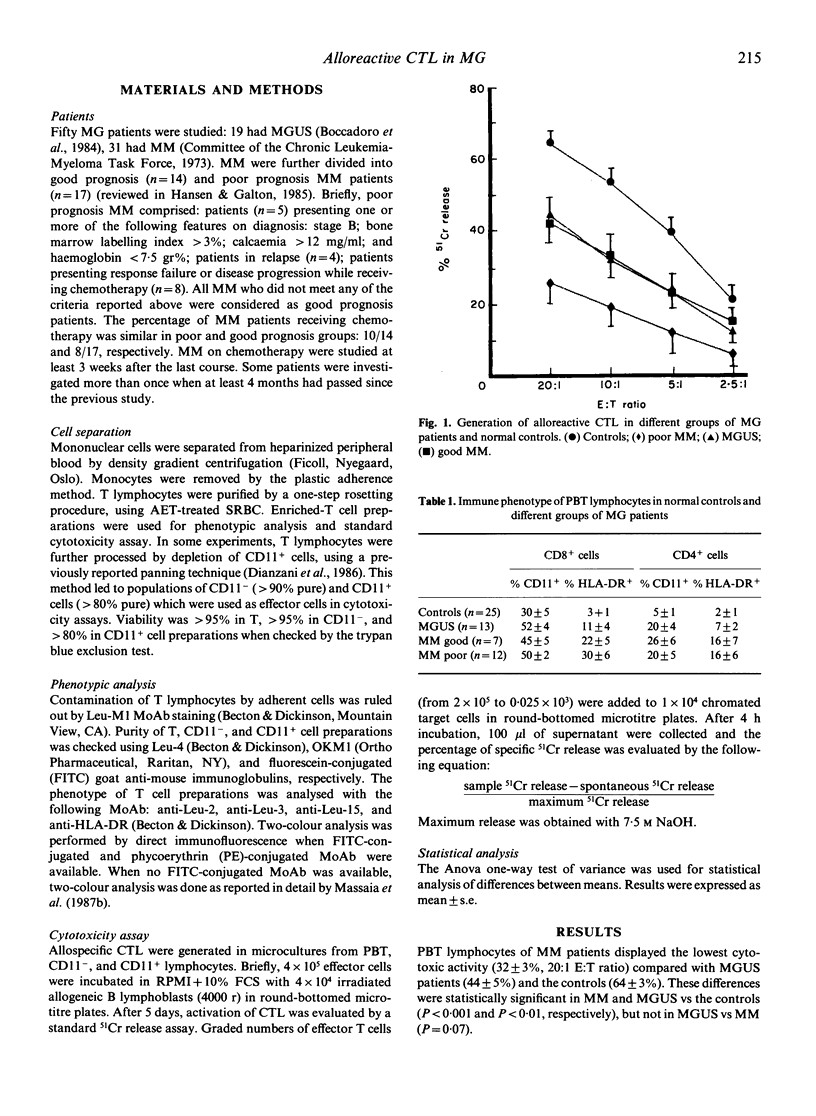
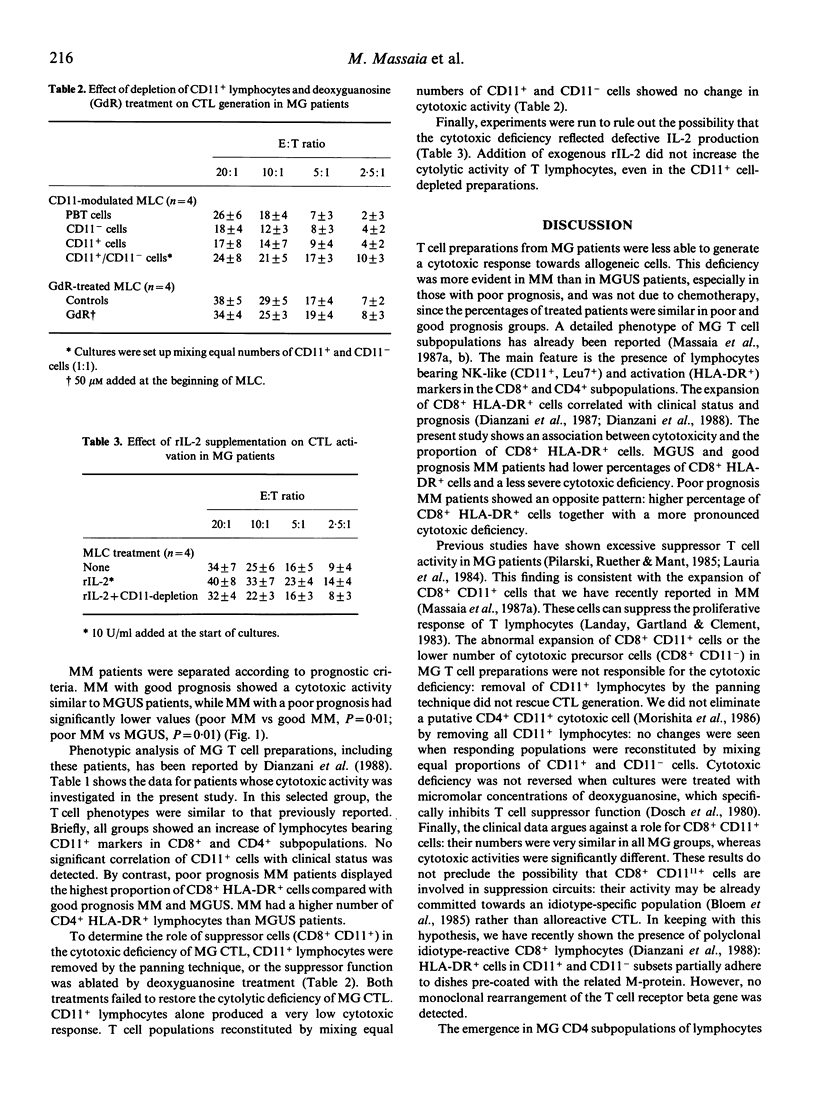
The generation of cytotoxic T lymphocytes (CTL) towards allogeneic cells was investigated in 19 patients with monoclonal gammopathy of undetermined significance (MGUS) and 31 patients with multiple myeloma (MM). This function was significantly decreased in all patients. The cytotoxic deficiency was more pronounced in MM with poor prognosis than MM with good prognosis and MGUS patients. A phenotypic analysis of PBT lymphocytes showed that poor prognosis MM also had the highest proportions of activated cells (HLA-DR+) in CD8+ subpopulations. CTL were generated after depletion of CD11+ lymphocytes (including suppressor cells) or after inhibition of suppressor function with deoxyguanosine. No increase of cytotoxicity was detected under these conditions. Exogenous supplementation of recombinant interleukin 2 (rIL-2) was also ineffective. These data indicate that MG PBT lymphocytes are unable to fully differentiate into CTL following allogeneic stimulation. This deficiency is most evident in MM patients already showing the poorest prognosis and the most altered T cell phenotype.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloem A. C., Heijnen C. J., Bast B. J., Ballieux R. E. T cells in B cell chronic lymphocytic leukemia. II. Lack of antigen-specific T suppressor cells and their progenitors. J Immunol. 1985 Dec;135(6):4261–4265. [PubMed] [Google Scholar]
- Boccadoro M., Gavarotti P., Fossati G., Pileri A., Marmont F., Neretto G., Gallamini A., Volta C., Tribalto M., Testa M. G. Low plasma cell 3(H) thymidine incorporation in monoclonal gammopathy of undetermined significance (MGUS), smouldering myeloma and remission phase myeloma: a reliable indicator of patients not requiring therapy. Br J Haematol. 1984 Dec;58(4):689–696. doi: 10.1111/j.1365-2141.1984.tb06116.x. [DOI] [PubMed] [Google Scholar]
- Carney W. P., Iacoviello V., Hirsch M. S. Functional properties of T lymphocytes and their subsets in cytomegalovirus mononucleosis. J Immunol. 1983 Jan;130(1):390–393. [PubMed] [Google Scholar]
- Commes T., Klein B., Jourdan M., Bataille R. Production of interleukin 2 in multiple myeloma. Clin Exp Immunol. 1986 Mar;63(3):533–540. [PMC free article] [PubMed] [Google Scholar]
- Dailey M. O., Pillemer E., Weissman I. L. Protection against syngeneic lymphoma by a long-lived cytotoxic T-cell clone. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5384–5387. doi: 10.1073/pnas.79.17.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dianzani U., Massaia M., Pileri A., Grossi C. E., Clement L. T. Differential expression of ecto-5' nucleotidase activity by functionally and phenotypically distinct subpopulations of human Leu-2+/T8+ lymphocytes. J Immunol. 1986 Jul 15;137(2):484–489. [PubMed] [Google Scholar]
- Dosch H. M., Mansour A., Cohen A., Shore A., Gelfand E. W. Inhibition of suppressor T-cell development following deoxyguanosine administration. Nature. 1980 Jun 12;285(5765):494–496. doi: 10.1038/285494a0. [DOI] [PubMed] [Google Scholar]
- Engers H. D., Sorenson G. D., Terres G., Horvath C., Brunner K. T. Functional activity in vivo of effector T cell populations. I. Antitumor activity exhibited by allogeneic mixed leukocyte culture cells. J Immunol. 1982 Sep;129(3):1292–1298. [PubMed] [Google Scholar]
- Hansen O. P., Galton D. A. Classification and prognostic variables in myelomatosis. Scand J Haematol. 1985 Jul;35(1):10–19. doi: 10.1111/j.1600-0609.1985.tb00792.x. [DOI] [PubMed] [Google Scholar]
- Landay A., Gartland G. L., Clement L. T. Characterization of a phenotypically distinct subpopulation of Leu-2+ cells that suppresses T cell proliferative responses. J Immunol. 1983 Dec;131(6):2757–2761. [PubMed] [Google Scholar]
- Lauria F., Foa R., Cavo M., Gobbi M., Raspadori D., Giubellino M. C., Tazzari P. L., Tura S. Membrane phenotype and functional behaviour of T lymphocytes in multiple myeloma: correlation with clinical stages of the disease. Clin Exp Immunol. 1984 Jun;56(3):653–658. [PMC free article] [PubMed] [Google Scholar]
- Massaia M., Dianzani U., Pioppo P., Sibilla E., Boccadoro M., Pileri A. Multiple myeloma: ecto-5' nucleotidase deficiency of suppressor/cytotoxic (CD8) lymphocytes is a marker for the expansion of suppressor T cells. Clin Exp Immunol. 1987 Aug;69(2):426–432. [PMC free article] [PubMed] [Google Scholar]
- Mellstedt H., Holm G. In vitro studies of lymphocytes from patients with plasma cell myeloma. I. Stimulation by mitogens and cytotoxic activities. Clin Exp Immunol. 1973 Nov;15(3):309–320. [PMC free article] [PubMed] [Google Scholar]
- Mellstedt H., Holm G., Pettersson D., Björkholm M., Johansson B., Lindemalm C., Peest D., Ahre A. T cells in monoclonal gammopathies. Scand J Haematol. 1982 Jul;29(1):57–64. doi: 10.1111/j.1600-0609.1982.tb00563.x. [DOI] [PubMed] [Google Scholar]
- Mills K. H., Cawley J. C. Abnormal monoclonal antibody-defined helper/suppressor T-cell subpopulations in multiple myeloma: relationship to treatment and clinical stage. Br J Haematol. 1983 Feb;53(2):271–275. doi: 10.1111/j.1365-2141.1983.tb02021.x. [DOI] [PubMed] [Google Scholar]
- Morell A., Terry W. D., Waldmann T. A. Metabolic properties of IgG subclasses in man. J Clin Invest. 1970 Apr;49(4):673–680. doi: 10.1172/JCI106279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita Y., Martin P. J., Bean M. A., Yamada H., Hansen J. A. Antigen-specific functions of a CD4+ subset of human T lymphocytes with granular morphology. J Immunol. 1986 Mar 15;136(6):2095–2102. [PubMed] [Google Scholar]
- Paglieroni T., Mackenzie M. R. Multiple myeloma: an immunologic profile. Cytotoxic and suppressive effects of the EA rosette-forming cell. J Immunol. 1980 Jun;124(6):2563–2570. [PubMed] [Google Scholar]
- Perri R. T., Oken M. M., Kay N. E. Enhanced T cell suppression is directed toward sensitive circulating B cells in multiple myeloma. J Lab Clin Med. 1982 Apr;99(4):512–519. [PubMed] [Google Scholar]
- Pilarski L. M., Ruether B. A., Mant M. J. Abnormal function of B lymphocytes from peripheral blood of multiple myeloma patients. Lack of correlation between the number of cells potentially able to secrete immunoglobulin M and serum immunoglobulin M levels. J Clin Invest. 1985 Jun;75(6):2024–2029. doi: 10.1172/JCI111921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platsoucas C. D., Hansen H. J., Redman J. R., Berenson S., Lee B. J., 3rd, Clarkson B. D. T-cell imbalances in patients with multiple myeloma: an analysis by monoclonal antibodies. J Clin Immunol. 1983 Jul;3(3):277–284. doi: 10.1007/BF00915352. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., O'Brien C., Rosenthal P., Schlossman S. F. The cellular basis for viral-induced immunodeficiency: analysis by monoclonal antibodies. J Immunol. 1980 Sep;125(3):1269–1274. [PubMed] [Google Scholar]
- Ullrich S., Zolla-Pazner S. Immunoregulatory circuits in myeloma. Clin Haematol. 1982 Feb;11(1):87–111. [PubMed] [Google Scholar]
- Velardi A., Mingari M. C., Moretta L., Grossi C. E. Functional analysis of cloned germinal center CD4+ cells with natural killer cell-related features. Divergence from typical T helper cells. J Immunol. 1986 Nov 1;137(9):2808–2813. [PubMed] [Google Scholar]



