Abstract
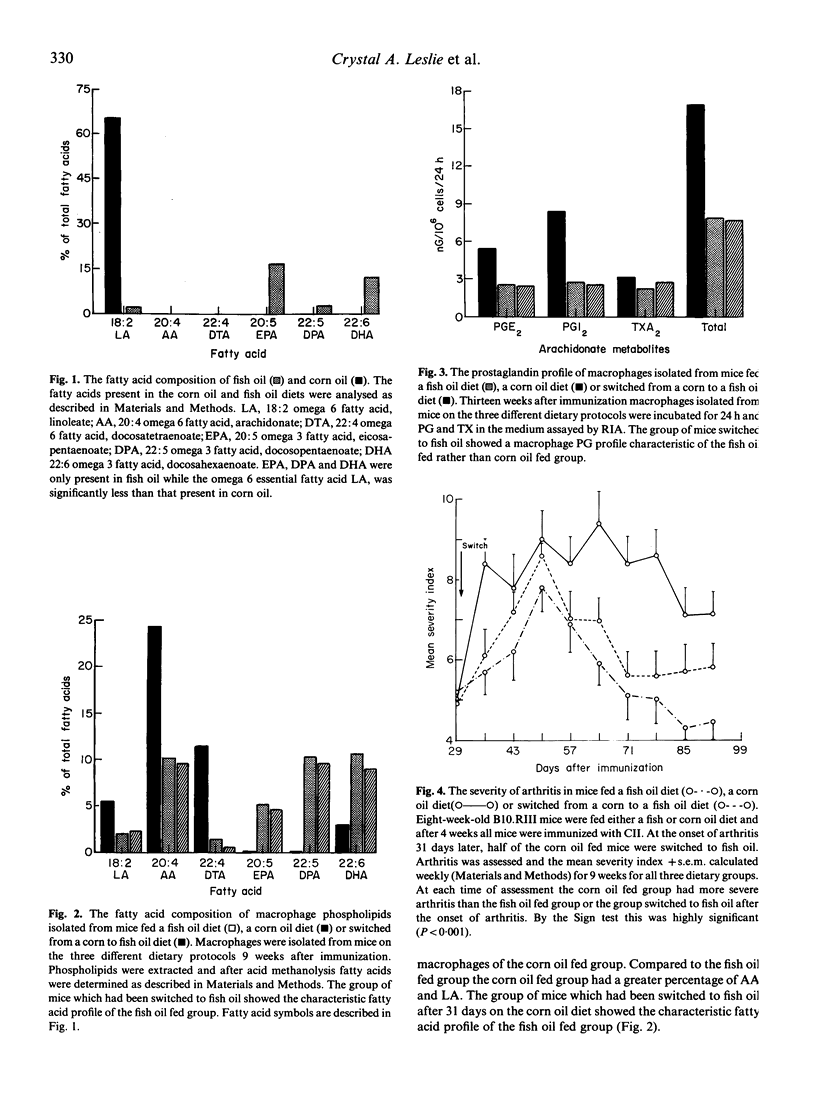
We have previously reported that compared to a corn oil diet a fish oil diet (5% by weight) fed to B10R.III mice before the induction of collagen induced arthritis markedly reduced disease severity. In this study we determine whether a fish oil diet could reduce the severity of collagen induced arthritis if begun after the arthritis was clinically apparent. Mice were initially fed either a fish oil or corn oil diet and immunized with bovine type II collagen 4 weeks later. At the onset of collagen-induced arthritis, half of the corn oil fed mice were switched to fish oil and arthritis assessed on a weekly basis. Four weeks after the diet change until killing 5 weeks later, the mice switched to fish oil developed much less severe arthritis than the corn oil fed controls. Thus the severity index of corn oil fed mice ranged between 9.4 and 7.1; the severity index of fish oil fed mice was between 6.8 and 4.3 while the mice switched to fish oil ranged between 7.2 and 5.6. Analysis of peritoneal macrophages 13 weeks after immunization showed that macrophages from fish oil fed mice incorporated eicosapentaenoic acid into phospholipids and produced less arachidonate products than corn oil fed mice. There was no difference between macrophages obtained from mice switched from corn oil to fish oil and those maintained on fish oil with respect to fatty acid composition of membrane phospholipids or prostaglandin profile. These results suggest that arthritis severity may be modulated after the onset of CIA by altering the PG profile of macrophages present at inflammatory sites.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cathcart E. S., Hayes K. C., Gonnerman W. A., Lazzari A. A., Franzblau C. Experimental arthritis in a nonhuman primate. I. Induction by bovine type II collagen. Lab Invest. 1986 Jan;54(1):26–31. [PubMed] [Google Scholar]
- Courtenay J. S., Dallman M. J., Dayan A. D., Martin A., Mosedale B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature. 1980 Feb 14;283(5748):666–668. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Goodwin J. S., Webb D. R. Regulation of the immune response by prostaglandins. Clin Immunol Immunopathol. 1980 Jan;15(1):106–122. doi: 10.1016/0090-1229(80)90024-0. [DOI] [PubMed] [Google Scholar]
- Griffiths M. M., DeWitt C. W. Genetic control of collagen-induced arthritis in rats: the immune response to type II collagen among susceptible and resistant strains and evidence for multiple gene control. J Immunol. 1984 Jun;132(6):2830–2836. [PubMed] [Google Scholar]
- Holmdahl R., Jansson L., Larsson E., Rubin K., Klareskog L. Homologous type II collagen induces chronic and progressive arthritis in mice. Arthritis Rheum. 1986 Jan;29(1):106–113. doi: 10.1002/art.1780290114. [DOI] [PubMed] [Google Scholar]
- Kelley V. E., Ferretti A., Izui S., Strom T. B. A fish oil diet rich in eicosapentaenoic acid reduces cyclooxygenase metabolites, and suppresses lupus in MRL-lpr mice. J Immunol. 1985 Mar;134(3):1914–1919. [PubMed] [Google Scholar]
- Kremer J. M., Bigauoette J., Michalek A. V., Timchalk M. A., Lininger L., Rynes R. I., Huyck C., Zieminski J., Bartholomew L. E. Effects of manipulation of dietary fatty acids on clinical manifestations of rheumatoid arthritis. Lancet. 1985 Jan 26;1(8422):184–187. doi: 10.1016/s0140-6736(85)92024-0. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Hoover R. L., Williams J. D., Sperling R. I., Ravalese J., 3rd, Spur B. W., Robinson D. R., Corey E. J., Lewis R. A., Austen K. F. Effect of dietary enrichment with eicosapentaenoic and docosahexaenoic acids on in vitro neutrophil and monocyte leukotriene generation and neutrophil function. N Engl J Med. 1985 May 9;312(19):1217–1224. doi: 10.1056/NEJM198505093121903. [DOI] [PubMed] [Google Scholar]
- Leitch A. G., Lee T. H., Ringel E. W., Prickett J. D., Robinson D. R., Pyne S. G., Corey E. J., Drazen J. M., Austen K. F., Lewis R. A. Immunologically induced generation of tetraene and pentaene leukotrienes in the peritoneal cavities of menhaden-fed rats. J Immunol. 1984 May;132(5):2559–2565. [PubMed] [Google Scholar]
- Leslie C. A., Gonnerman W. A., Cathcart E. S. Gender differences in eicosanoid production from macrophages of arthritis-susceptible mice. J Immunol. 1987 Jan 15;138(2):413–416. [PubMed] [Google Scholar]
- Leslie C. A., Gonnerman W. A., Ullman M. D., Hayes K. C., Franzblau C., Cathcart E. S. Dietary fish oil modulates macrophage fatty acids and decreases arthritis susceptibility in mice. J Exp Med. 1985 Oct 1;162(4):1336–1349. doi: 10.1084/jem.162.4.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie C. A., Pavlakis A. J., Wheeler J. S., Jr, Siroky M. B., Krane R. J. Release of arachidonate cascade products by the rabbit bladder; neurophysiological significance? J Urol. 1984 Aug;132(2):376–379. doi: 10.1016/s0022-5347(17)49631-5. [DOI] [PubMed] [Google Scholar]
- Prickett J. D., Trentham D. E., Robinson D. R. Dietary fish oil augments the induction of arthritis in rats immunized with type II collagen. J Immunol. 1984 Feb;132(2):725–729. [PubMed] [Google Scholar]
- Robinson D. R., Prickett J. D., Makoul G. T., Steinberg A. D., Colvin R. B. Dietary fish oil reduces progression of established renal disease in (NZB x NZW)F1 mice and delays renal disease in BXSB and MRL/1 strains. Arthritis Rheum. 1986 Apr;29(4):539–546. doi: 10.1002/art.1780290412. [DOI] [PubMed] [Google Scholar]
- Stuart J. M., Cremer M. A., Townes A. S., Kang A. H. Type II collagen-induced arthritis in rats. Passive transfer with serum and evidence that IgG anticollagen antibodies can cause arthritis. J Exp Med. 1982 Jan 1;155(1):1–16. doi: 10.1084/jem.155.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. E., Townes A. S., Kang A. H. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977 Sep 1;146(3):857–868. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance D. E., Sweeley C. C. Quantitative determination of the neutral glycosyl ceramides in human blood. J Lipid Res. 1967 Nov;8(6):621–630. [PubMed] [Google Scholar]
- Wooley P. H., Luthra H. S., Stuart J. M., David C. S. Type II collagen-induced arthritis in mice. I. Major histocompatibility complex (I region) linkage and antibody correlates. J Exp Med. 1981 Sep 1;154(3):688–700. doi: 10.1084/jem.154.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziff M. Diet in the treatment of rheumatoid arthritis. Arthritis Rheum. 1983 Apr;26(4):457–461. doi: 10.1002/art.1780260402. [DOI] [PubMed] [Google Scholar]


