Abstract
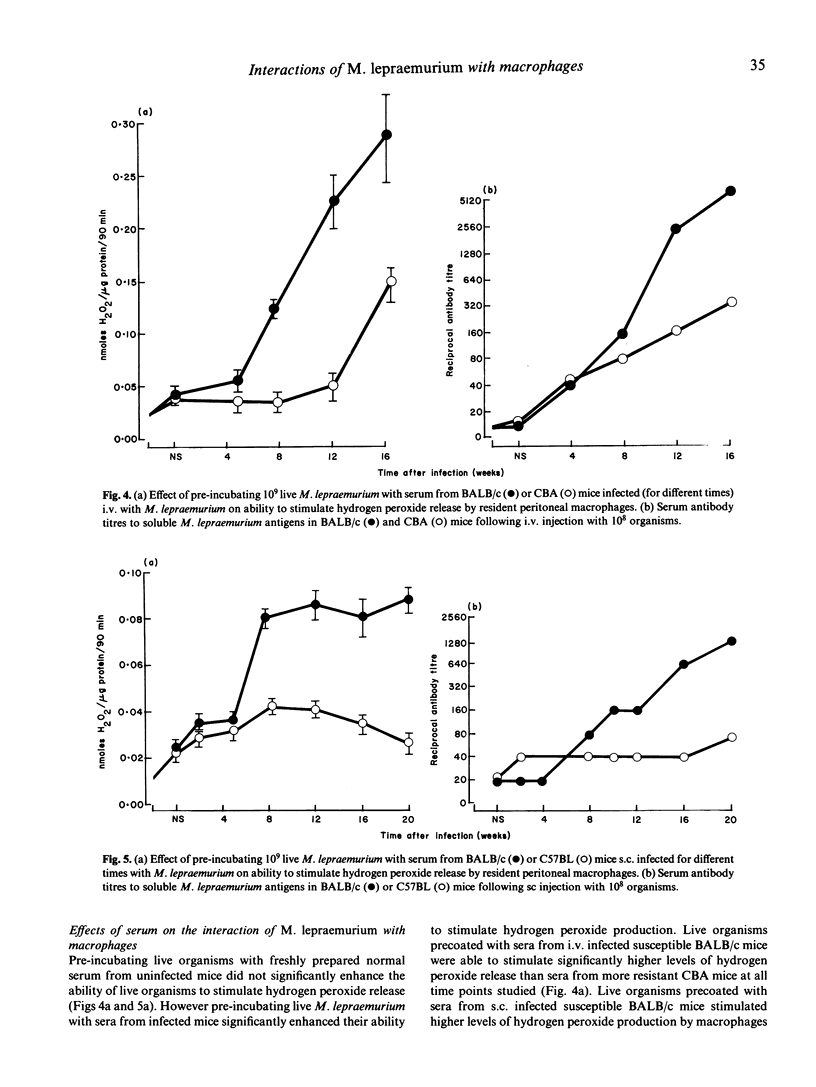
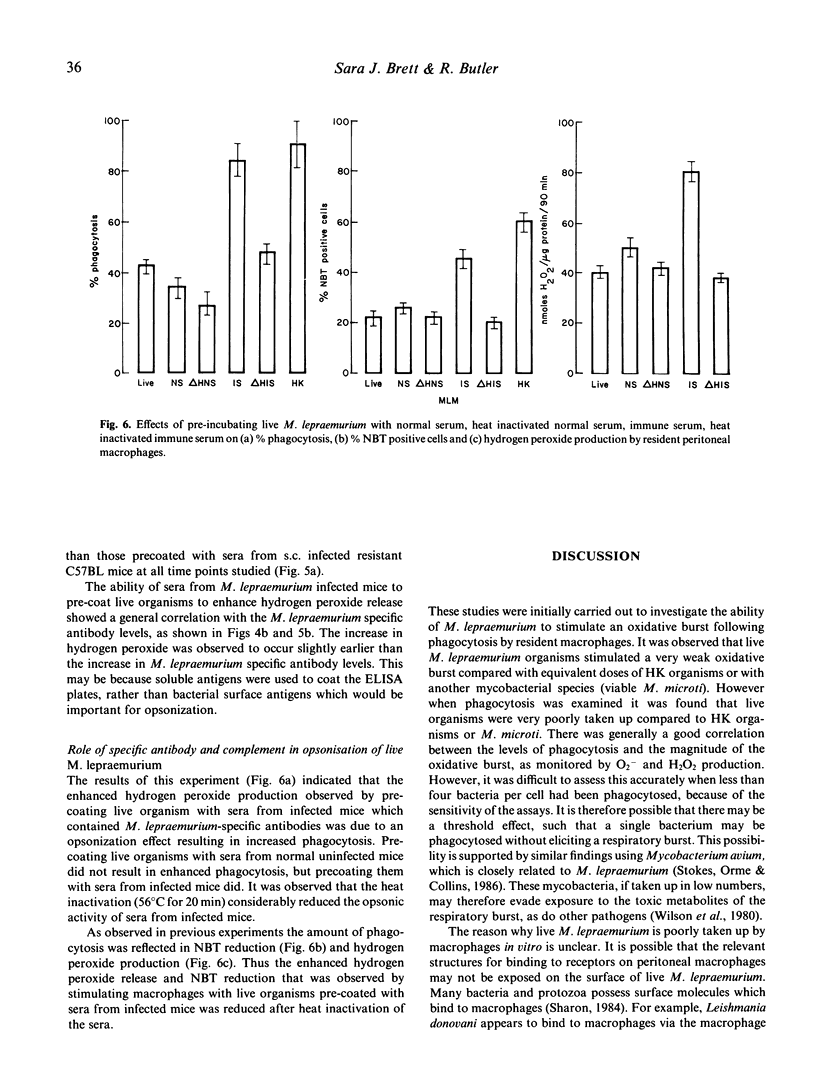
Live Mycobacterium lepraemurium or 60Co-gamma-irradiated organisms stimulated a very weak oxidative burst compared with similar numbers of heat-killed organisms or with live M. microti. This was found to reflect the poor uptake of the living organisms rather than an absolute failure to stimulate an oxidative burst. It is possible, however, that phagocytosis of fewer than 3-4 bacteria may not trigger the respiratory burst. Pre-incubating live M. lepraemurium with sera from infected mice, but not with fresh normal mouse sera, resulted in enhanced phagocytosis with a concomitant increase in the oxidative burst. The level of opsonic activity correlated with the M. lepraemurium specific antibody titres. The opsonic activity appeared to be mediated by antigen-antibody activation of the classical complement pathway as heat-inactivation destroyed the activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackwell J. M., Ezekowitz R. A., Roberts M. B., Channon J. Y., Sim R. B., Gordon S. Macrophage complement and lectin-like receptors bind Leishmania in the absence of serum. J Exp Med. 1985 Jul 1;162(1):324–331. doi: 10.1084/jem.162.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P. J. Structures of the typing antigens of atypical mycobacteria: a brief review of present knowledge. Rev Infect Dis. 1981 Sep-Oct;3(5):905–913. doi: 10.1093/clinids/3.5.905. [DOI] [PubMed] [Google Scholar]
- Brett S. J., Payne S. N., Gigg J., Burgess P., Gigg R. Use of synthetic glycoconjugates containing the Mycobacterium leprae specific and immunodominant epitope of phenolic glycolipid I in the serology of leprosy. Clin Exp Immunol. 1986 Jun;64(3):476–483. [PMC free article] [PubMed] [Google Scholar]
- Brett S. J. T-cell responsiveness in Mycobacterium lepraemurium infections in a "resistant" (CBA) and a "susceptible" (BALB/c) mouse strain. Cell Immunol. 1984 Nov;89(1):132–143. doi: 10.1016/0008-8749(84)90204-1. [DOI] [PubMed] [Google Scholar]
- Brown I. N., Glynn A. A., Plant J. Inbred mouse strain resistance to Mycobacterium lepraemurium follows the Ity/Lsh pattern. Immunology. 1982 Sep;47(1):149–156. [PMC free article] [PubMed] [Google Scholar]
- Curtis J., Adu H. O., Turk J. L. H-2 linkage control of resistance to subcutaneous infection with Mycobacterium lepraemurium. Infect Immun. 1982 Nov;38(2):434–439. doi: 10.1128/iai.38.2.434-439.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper P., Rees R. J. The nature of the electron-transparent zone that surrounds Mycobacterium lepraemurium inside host cells. J Gen Microbiol. 1973 Jul;77(1):79–87. doi: 10.1099/00221287-77-1-79. [DOI] [PubMed] [Google Scholar]
- Gangadharam P. R., Pratt P. E. Susceptibility of Mycobacterium intracellulare to hydrogen peroxide. Am Rev Respir Dis. 1984 Aug;130(2):309–311. doi: 10.1164/arrd.1984.130.2.309. [DOI] [PubMed] [Google Scholar]
- HART P. D., REES R. J. Effect of macrocyclon in acute and chronic pulmonary tuberculous infection in mice as shown by viable and total bacterial counts. Br J Exp Pathol. 1960 Aug;41:414–421. [PMC free article] [PubMed] [Google Scholar]
- Hart P. D., Armstrong J. A., Brown C. A., Draper P. Ultrastructural study of the behavior of macrophages toward parasitic mycobacteria. Infect Immun. 1972 May;5(5):803–807. doi: 10.1128/iai.5.5.803-807.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer T. J., Nelson K. E., Crispen R. G., Andersen B. R. Mycobacterium leprae fails to stimulate phagocytic cell superoxide anion generation. Infect Immun. 1986 Feb;51(2):514–520. doi: 10.1128/iai.51.2.514-520.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor N. Intracellular location of Mycobacterium leprae in macrophages of normal and immune-deficient mice and effect of rifampin. Infect Immun. 1983 Nov;42(2):802–811. doi: 10.1128/iai.42.2.802-811.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. III. Enhanced oxidative metabolism as an expression of macrophage activation. J Exp Med. 1980 Dec 1;152(6):1596–1609. doi: 10.1084/jem.152.6.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F. Mechanisms of macrophage antimicrobial activity. Trans R Soc Trop Med Hyg. 1983;77(5):620–630. doi: 10.1016/0035-9203(83)90190-6. [DOI] [PubMed] [Google Scholar]
- Ramanathan V. D., Curtis J., Turk J. L. Activation of the alternative pathway of complement by mycobacteria and cord factor. Infect Immun. 1980 Jul;29(1):30–35. doi: 10.1128/iai.29.1.30-35.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruch W., Cooper P. H., Baggiolini M. Assay of H2O2 production by macrophages and neutrophils with homovanillic acid and horse-radish peroxidase. J Immunol Methods. 1983 Oct 28;63(3):347–357. doi: 10.1016/s0022-1759(83)80008-8. [DOI] [PubMed] [Google Scholar]
- Sharp A. K., Colston M. J., Banerjee D. K. Susceptibility of Mycobacterium leprae to the bactericidal activity of mouse peritoneal macrophages and to hydrogen peroxide. J Med Microbiol. 1985 Feb;19(1):77–84. doi: 10.1099/00222615-19-1-77. [DOI] [PubMed] [Google Scholar]
- Van Dijk W. C., Verbrugh H. A., van der Tol M. E., Peters R., Verhoef J. Role of Escherichia coli K capsular antigens during complement activation, C3 fixation, and opsonization. Infect Immun. 1979 Aug;25(2):603–609. doi: 10.1128/iai.25.2.603-609.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugh H. A., Lee D. A., Elliott G. R., Keane W. F., Hoidal J. R., Peterson P. K. Opsonization of Legionella pneumophila in human serum: key roles for specific antibodies and the classical complement pathway. Immunology. 1985 Apr;54(4):643–653. [PMC free article] [PubMed] [Google Scholar]
- Wheeler P. R., Gregory D. Superoxide dismutase, peroxidatic activity and catalase in Mycobacterium leprae purified from armadillo liver. J Gen Microbiol. 1980 Dec;121(2):457–464. doi: 10.1099/00221287-121-2-457. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Tsai V., Remington J. S. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J Exp Med. 1980 Feb 1;151(2):328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Silverstein S. C. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J Exp Med. 1983 Dec 1;158(6):2016–2023. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]


