Abstract
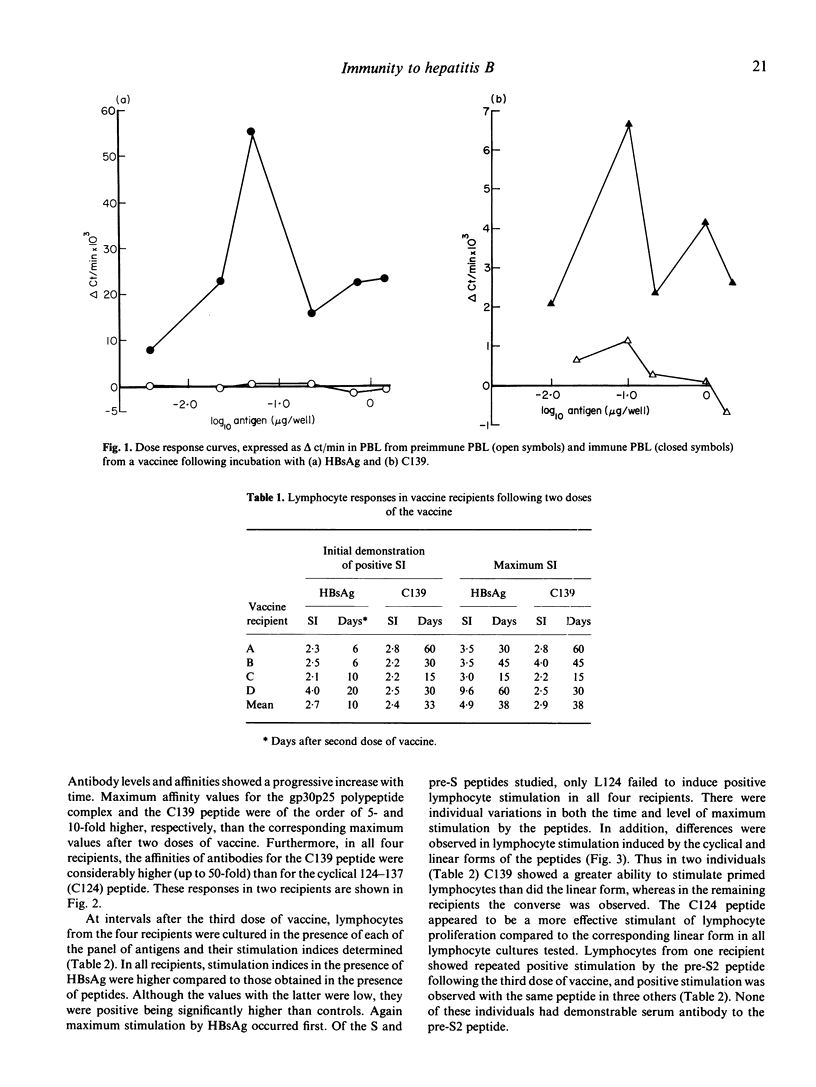
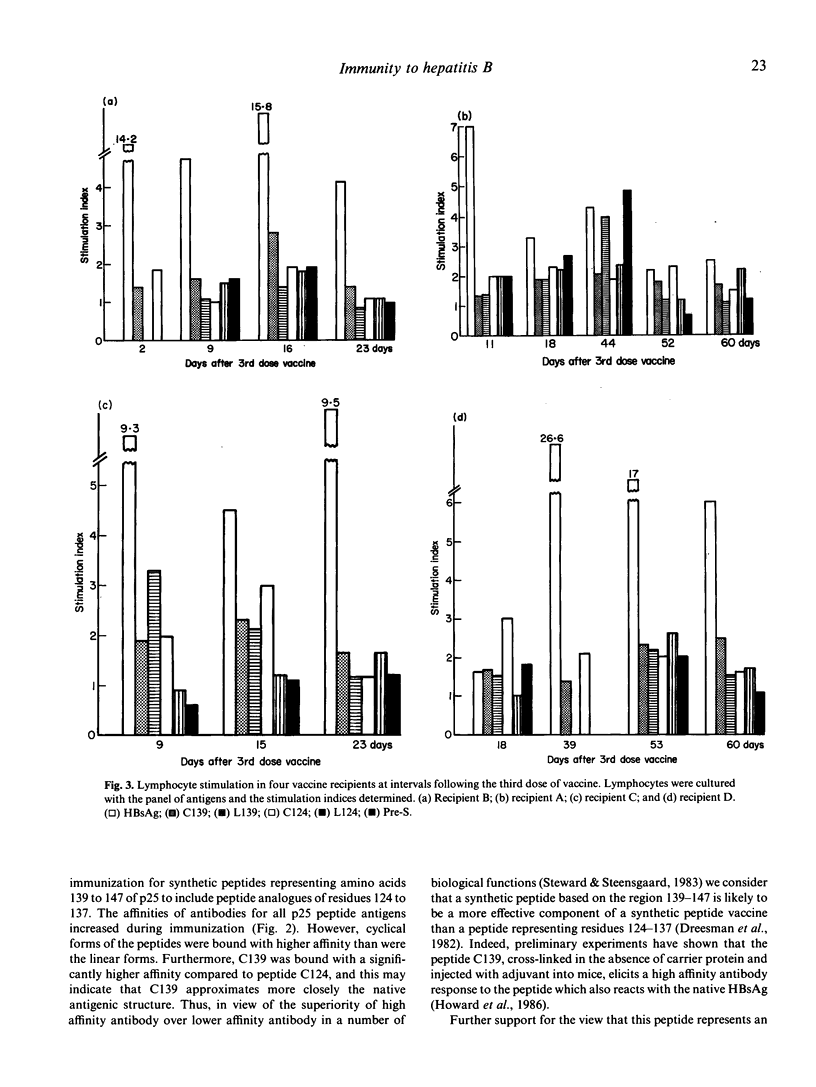
The potential of a panel of synthetic HBsAg peptides as components of a synthetic hepatitis B vaccine was assessed. Each was used in turn as probes to analyse human immune responses to a licensed plasma-derived HBV vaccine. Both humoral and cellular responses were analysed with synthetic peptides representing residues 124-147 of the surface antigen of the virus (HBsAg) and residues 126-140 of the pre-S2 region. Antibody levels and affinities were assessed in radioimmunoassays with synthetic linear and cyclical forms of surface antigen peptides 124-137 and 139-147, with the gp30p25 polypeptide complex of HBsAg and with the linear pre-S2 peptide 126-140. Levels and affinities of antibodies to the antigens increased with time during immunization. However, antibodies binding the cyclical peptide representing amino acids 139 to 147 (C139) were present at higher levels and had higher affinities than were antibodies binding the other peptides, indicating that C139 more closely approximates a domain on the native antigen than do the other peptides. No humoral responses were measured with the pre-S2 peptide. Cellular responses were assessed by in vitro stimulation of peripheral blood lymphocytes by HBsAg and by the synthetic peptides. All vaccine recipients had demonstrable lymphocyte responsiveness to HBsAg after both second and third doses of the vaccine. Of the S and pre-S peptides used, only L124 failed to induce lymphocyte stimulation in all recipients. However, there were individual variations in both the time of initial responsiveness to peptides and in the level and time of maximal stimulation. Stimulation by native HBsAg particles, which corresponded to the appearance of anti-HBs antibody, preceded that observed using synthetic peptides. In all recipients, maximum stimulation indices with HBsAg were significantly higher than those observed with the peptides. In contrast to the absence of pre-S2 antibody, the lymphocytes from all recipients showed positive stimulation in response to the peptide representing residues 126-140 of the pre-S2 region. None of these individuals had antibodies to pre-S or an HB core peptide sequence nor did their lymphocytes respond to a synthetic peptide representing an HB core sequence.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhatnagar P. K., Papas E., Blum H. E., Milich D. R., Nitecki D., Karels M. J., Vyas G. N. Immune response to synthetic peptide analogues of hepatitis B surface antigen specific for the a determinant. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4400–4404. doi: 10.1073/pnas.79.14.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. E., Howard C. R., Zuckerman A. J., Steward M. W. Affinity of antibody responses in man to hepatitis B vaccine determined with synthetic peptides. Lancet. 1984 Jul 28;2(8396):184–187. doi: 10.1016/s0140-6736(84)90479-3. [DOI] [PubMed] [Google Scholar]
- Brown S. E., Howard C. R., Zuckerman A. J., Steward M. W. Determination of the affinity of antibodies to hepatitis B surface antigen in human sera. J Immunol Methods. 1984 Aug 3;72(1):41–48. doi: 10.1016/0022-1759(84)90431-9. [DOI] [PubMed] [Google Scholar]
- DeLisi C., Berzofsky J. A. T-cell antigenic sites tend to be amphipathic structures. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7048–7052. doi: 10.1073/pnas.82.20.7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreesman G. R., Sanchez Y., Ionescu-Matiu I., Sparrow J. T., Six H. R., Peterson D. L., Hollinger F. B., Melnick J. L. Antibody to hepatitis B surface antigen after a single inoculation of uncoupled synthetic HBsAg peptides. Nature. 1982 Jan 14;295(5845):158–160. doi: 10.1038/295158a0. [DOI] [PubMed] [Google Scholar]
- Gerin J. L., Alexander H., Shih J. W., Purcell R. H., Dapolito G., Engle R., Green N., Sutcliffe J. G., Shinnick T. M., Lerner R. A. Chemically synthesized peptides of hepatitis B surface antigen duplicate the d/y specificities and induce subtype-specific antibodies in chimpanzees. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2365–2369. doi: 10.1073/pnas.80.8.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R. G., Hoofnagle J. H., Minuk G. Y., Purcell R. H., Gerin J. L. Cell-mediated immunity to hepatitis B surface antigen in man. Clin Exp Immunol. 1984 Aug;57(2):257–264. [PMC free article] [PubMed] [Google Scholar]
- Imai M., Goto A., Nishioka K., Kurashina S., Miyakawa Y. Antigenicity of reduced and alkylated Australia antigen. J Immunol. 1974 Jan;112(1):416–419. [PubMed] [Google Scholar]
- Lerner R. A., Green N., Alexander H., Liu F. T., Sutcliffe J. G., Shinnick T. M. Chemically synthesized peptides predicted from the nucleotide sequence of the hepatitis B virus genome elicit antibodies reactive with the native envelope protein of Dane particles. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3403–3407. doi: 10.1073/pnas.78.6.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich D. R., Peterson D. L., Leroux-Roels G. G., Lerner R. A., Chisari F. V. Genetic regulation of the immune response to hepatitis B surface antigen (HBsAg). VI. T cell fine specificity. J Immunol. 1985 Jun;134(6):4203–4211. [PubMed] [Google Scholar]
- Neurath A. R., Kent S. B., Parker K., Prince A. M., Strick N., Brotman B., Sproul P. Antibodies to a synthetic peptide from the preS 120-145 region of the hepatitis B virus envelope are virus neutralizing. Vaccine. 1986 Mar;4(1):35–37. doi: 10.1016/s0264-410x(86)80001-9. [DOI] [PubMed] [Google Scholar]
- Neurath A. R., Kent S. B., Strick N. Detection of antiviral antibodies with predetermined specificity using synthetic peptide--beta-lactamase conjugates: application to antibodies specific for the preS region of the hepatitis B virus envelope proteins. J Gen Virol. 1986 Mar;67(Pt 3):453–461. doi: 10.1099/0022-1317-67-3-453. [DOI] [PubMed] [Google Scholar]
- Neurath A. R., Kent S. B., Strick N., Taylor P., Stevens C. E. Hepatitis B virus contains pre-S gene-encoded domains. Nature. 1985 May 9;315(6015):154–156. doi: 10.1038/315154a0. [DOI] [PubMed] [Google Scholar]
- Skelly J., Howard C. R., Zuckerman A. J. Analysis of hepatitis B surface antigen components solubilized with Triton X-100. J Gen Virol. 1979 Sep;44(3):679–689. doi: 10.1099/0022-1317-44-3-679. [DOI] [PubMed] [Google Scholar]
- Swenson P. D., Escobar M. R., Carithers R. L., Jr, Sobieski T. J., 3rd Failure of preexisting antibody against hepatitis B surface antigen to prevent subsequent hepatitis B infection. J Clin Microbiol. 1983 Aug;18(2):305–309. doi: 10.1128/jcm.18.2.305-309.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvan S. P., Hellström U. B., Lundbergh P. R. Detection of cellular and humoral immunity to hepatitis B surface antigen (HBsAg) in asymptomatic HBsAg carriers. Clin Exp Immunol. 1985 Nov;62(2):288–295. [PMC free article] [PubMed] [Google Scholar]
- Szmuness W., Stevens C. E., Harley E. J., Zang E. A., Alter H. J., Taylor P. E., DeVera A., Chen G. T., Kellner A. Hepatitis B vaccine in medical staff of hemodialysis units: efficacy and subtype cross-protection. N Engl J Med. 1982 Dec 9;307(24):1481–1486. doi: 10.1056/NEJM198212093072403. [DOI] [PubMed] [Google Scholar]
- Szmuness W., Stevens C. E., Harley E. J., Zang E. A., Oleszko W. R., William D. C., Sadovsky R., Morrison J. M., Kellner A. Hepatitis B vaccine: demonstration of efficacy in a controlled clinical trial in a high-risk population in the United States. N Engl J Med. 1980 Oct 9;303(15):833–841. doi: 10.1056/NEJM198010093031501. [DOI] [PubMed] [Google Scholar]
- Thanavala Y. M., Brown S. E., Howard C. R., Roitt I. M., Steward M. W. A surrogate hepatitis B virus antigenic epitope represented by a synthetic peptide and an internal image antiidiotype antibody. J Exp Med. 1986 Jul 1;164(1):227–236. doi: 10.1084/jem.164.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P., Vaudin M., Dixon J., Zuckerman A. J. Preparation of hepatitis B polypeptide micelles from human carrier plasma. J Virol Methods. 1982 Apr;4(3):177–185. doi: 10.1016/0166-0934(82)90046-5. [DOI] [PubMed] [Google Scholar]


