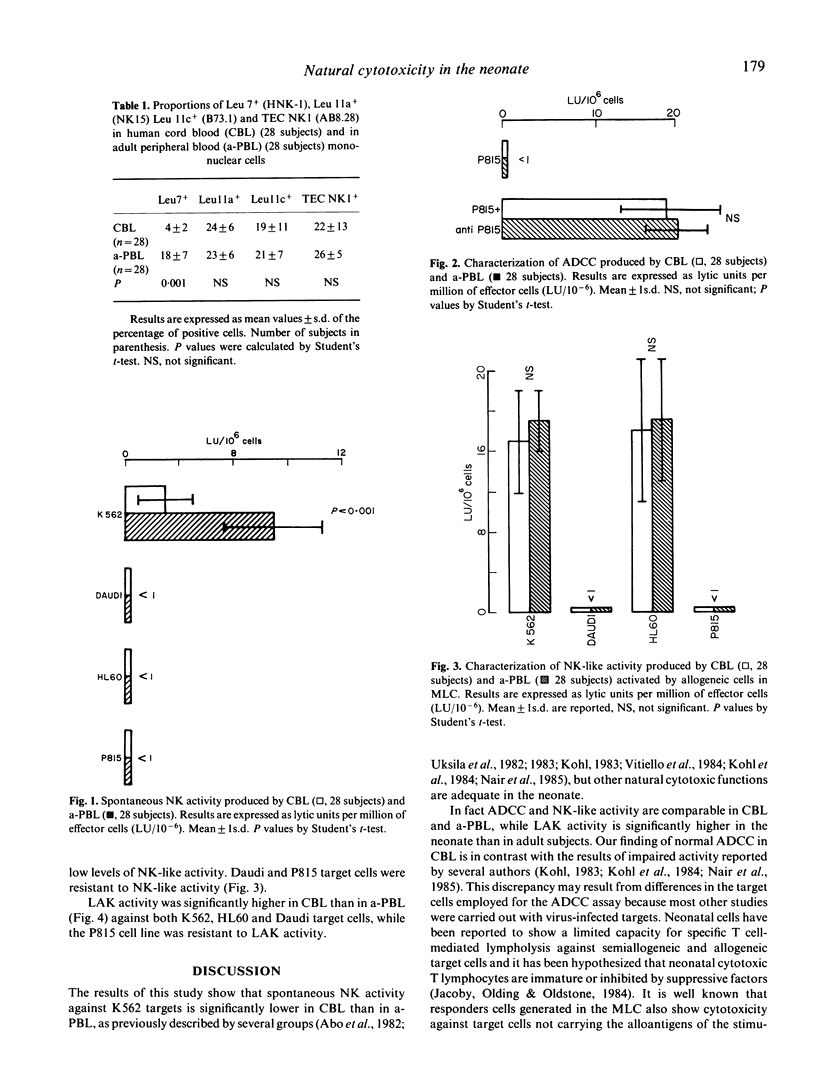
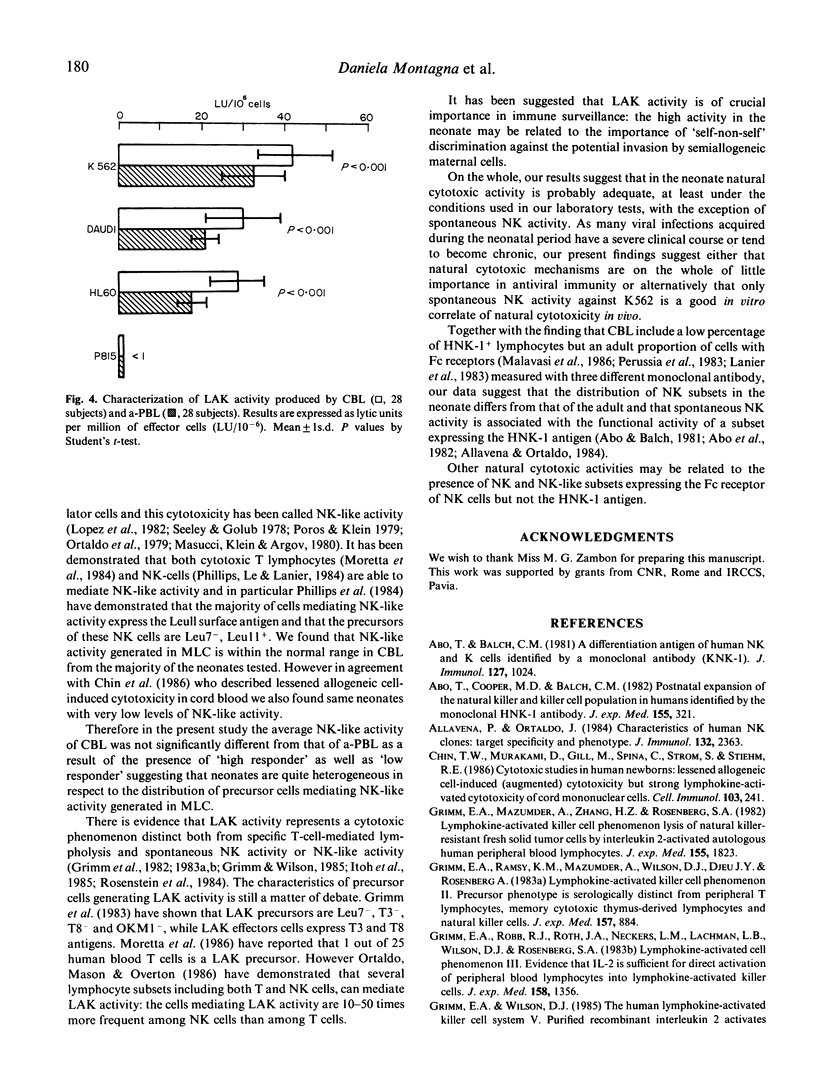
Abstract
In 28 healthy full-term newborns the percentage of circulating cells expressing the Leu7 antigen, the marker of natural killer (NK) cells, was significantly lower than in healthy adults. However, newborns and adults did not differ with regard to the percentage of cells reacting with the Leulla, Leullc and TEC NK-1, monoclonal antibodies directed against the IgG Fc receptor of killer cells. Spontaneous NK activity of neonatal cells was profoundly reduced compared to the adult. In contrast, antibody dependent cellular cytotoxicity and NK-like activity generated in mixed lymphocyte cultures were similar in the two groups and lymphokine-activated killer cell (LAK) activity was high in the neonate. Natural killing is thought to play an important role in antiviral immunity since the neonate has a deficient capacity to deal with viral infections. Consequently, the present data indicate either that spontaneous NK is the most informative in vitro measure of newborn natural cytotoxicity in vivo, or, alternatively, that natural killing is not as important in antiviral immunity as previously suggested.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Balch C. M. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1). J Immunol. 1981 Sep;127(3):1024–1029. [PubMed] [Google Scholar]
- Abo T., Cooper M. D., Balch C. M. Postnatal expansion of the natural killer and keller cell population in humans identified by the monoclonal HNK-1 antibody. J Exp Med. 1982 Jan 1;155(1):321–326. doi: 10.1084/jem.155.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allavena P., Ortaldo J. R. Characteristics of human NK clones: target specificity and phenotype. J Immunol. 1984 May;132(5):2363–2369. [PubMed] [Google Scholar]
- Chin T. W., Ank B. J., Murakami D., Gill M., Spina C., Strom S., Stiehm E. R. Cytotoxic studies in human newborns: lessened allogeneic cell-induced (augmented) cytotoxicity but strong lymphokine-activated cytotoxicity of cord mononuclear cells. Cell Immunol. 1986 Dec;103(2):241–251. doi: 10.1016/0008-8749(86)90087-0. [DOI] [PubMed] [Google Scholar]
- Grimm E. A., Mazumder A., Zhang H. Z., Rosenberg S. A. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982 Jun 1;155(6):1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm E. A., Robb R. J., Roth J. A., Neckers L. M., Lachman L. B., Wilson D. J., Rosenberg S. A. Lymphokine-activated killer cell phenomenon. III. Evidence that IL-2 is sufficient for direct activation of peripheral blood lymphocytes into lymphokine-activated killer cells. J Exp Med. 1983 Oct 1;158(4):1356–1361. doi: 10.1084/jem.158.4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm E. A., Wilson D. J. The human lymphokine-activated killer cell system. V. Purified recombinant interleukin 2 activates cytotoxic lymphocytes which lyse both natural killer-resistant autologous and allogeneic tumors and trinitrophenyl-modified autologous peripheral blood lymphocytes. Cell Immunol. 1985 Sep;94(2):568–578. doi: 10.1016/0008-8749(85)90280-1. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Holden H. T. Natural cell-mediated immunity. Adv Cancer Res. 1978;27:305–377. doi: 10.1016/s0065-230x(08)60936-7. [DOI] [PubMed] [Google Scholar]
- Itoh K., Tilden A. B., Kumagai K., Balch C. M. Leu-11+ lymphocytes with natural killer (NK) activity are precursors of recombinant interleukin 2 (rIL 2)-induced activated killer (AK) cells. J Immunol. 1985 Feb;134(2):802–807. [PubMed] [Google Scholar]
- Jacoby D. R., Olding L. B., Oldstone M. B. Immunologic regulation of fetal-maternal balance. Adv Immunol. 1984;35:157–208. doi: 10.1016/s0065-2776(08)60576-3. [DOI] [PubMed] [Google Scholar]
- Kohl S. Defective infant antiviral cytotoxicity to herpes simplex virus-infected cells. J Pediatr. 1983 Jun;102(6):885–888. doi: 10.1016/s0022-3476(83)80019-5. [DOI] [PubMed] [Google Scholar]
- Kohl S., Loo L. S., Gonik B. Analysis in human neonates of defective antibody-dependent cellular cytotoxicity and natural killer cytotoxicity to herpes simplex virus-infected cells. J Infect Dis. 1984 Jul;150(1):14–19. doi: 10.1093/infdis/150.1.14. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Phillips J. H., Warner N. L., Babcock G. F. Subpopulations of human natural killer cells defined by expression of the Leu-7 (HNK-1) and Leu-11 (NK-15) antigens. J Immunol. 1983 Oct;131(4):1789–1796. [PubMed] [Google Scholar]
- López-Botet M., Silva A., Rodríguez J., de Landazuri M. O. Generation of T cell blasts with NK-like activity in human MLC: cellular precursors, IL 2 responsiveness, and phenotype expression. J Immunol. 1982 Sep;129(3):1109–1115. [PubMed] [Google Scholar]
- Maccario R., Nespoli L., Mingrat G., Vitiello A., Ugazio A. G., Burgio G. R. Lymphocyte subpopulations in the neonate: identification of an immature subset of OKT8-positive, OKT3-negative cells. J Immunol. 1983 Mar;130(3):1129–1131. [PubMed] [Google Scholar]
- Masucci M. G., Klein E., Argov S. Disappearance of the NK effect after explantation of lymphocytes and generation of similar nonspecific cytotoxicity correlated to the level of blastogenesis in activated cultures. J Immunol. 1980 May;124(5):2458–2463. [PubMed] [Google Scholar]
- Moretta A., Pantaleo G., Mingari M. C., Melioli G., Moretta L., Cerottini J. C. Assignment of human natural killer (NK)-like cells to the T cell lineage. Single allospecific T cell clones lyse specific or NK-sensitive target cells via distinct recognition structures. Eur J Immunol. 1984 Feb;14(2):121–125. doi: 10.1002/eji.1830140204. [DOI] [PubMed] [Google Scholar]
- Moretta L., Pende D., Cozzani R., Merli A., Bagnasco M., Mingari M. C. T cell nature of some lymphokine-activated killer (LAK) cells. Frequency analysis of LAK precursors within human T cell populations and clonal analysis of LAK effector cells. Eur J Immunol. 1986 Dec;16(12):1623–1625. doi: 10.1002/eji.1830161224. [DOI] [PubMed] [Google Scholar]
- Nair M. P., Schwartz S. A., Menon M. Association of decreased natural and antibody-dependent cellular cytotoxicity and production of natural killer cytotoxic factor and interferon in neonates. Cell Immunol. 1985 Aug;94(1):159–171. doi: 10.1016/0008-8749(85)90093-0. [DOI] [PubMed] [Google Scholar]
- Ortaldo J. R., Bonnard G. D., Kind P. D., Herberman R. B. Cytotoxicity by cultured human lymphocytes: characteristics of effector cells and specificity of cytotoxicity. J Immunol. 1979 Apr;122(4):1489–1494. [PubMed] [Google Scholar]
- Ortaldo J. R., Mason A., Overton R. Lymphokine-activated killer cells. Analysis of progenitors and effectors. J Exp Med. 1986 Oct 1;164(4):1193–1205. doi: 10.1084/jem.164.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perussia B., Starr S., Abraham S., Fanning V., Trinchieri G. Human natural killer cells analyzed by B73.1, a monoclonal antibody blocking Fc receptor functions. I. Characterization of the lymphocyte subset reactive with B73.1. J Immunol. 1983 May;130(5):2133–2141. [PubMed] [Google Scholar]
- Phillips J. H., Le A. M., Lanier L. L. Natural killer cells activated in a human mixed lymphocyte response culture identified by expression of Leu-11 and class II histocompatibility antigens. J Exp Med. 1984 Apr 1;159(4):993–1008. doi: 10.1084/jem.159.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poros A., Klein E. Distinction of anti-K562 and anti-allocytotoxicity in in vitro-stimulated populations of human lymphocytes. Cell Immunol. 1979 Aug;46(1):57–68. doi: 10.1016/0008-8749(79)90245-4. [DOI] [PubMed] [Google Scholar]
- Rabin H., Hopkins R. F., 3rd, Ruscetti F. W., Neubauer R. H., Brown R. L., Kawakami T. G. Spontaneous release of a factor with properties of T cell growth factor from a continuous line of primate tumor T cells. J Immunol. 1981 Nov;127(5):1852–1856. [PubMed] [Google Scholar]
- Rosenstein M., Yron I., Kaufmann Y., Rosenberg S. A. Lymphokine-activated killer cells: lysis of fresh syngeneic natural killer-resistant murine tumor cells by lymphocytes cultured in interleukin 2. Cancer Res. 1984 May;44(5):1946–1953. [PubMed] [Google Scholar]
- Seeley J. K., Golub S. H. Studies on cytotoxicity generated in human mixed lymphocyte cultures. I. Time course and target spectrum of several distinct concomitant cytotoxic activities. J Immunol. 1978 Apr;120(4):1415–1422. [PubMed] [Google Scholar]
- Uksila J., Lassila O., Hirvonen T. Natural killer cell function of human neonatal lymphocytes. Clin Exp Immunol. 1982 Jun;48(3):649–654. [PMC free article] [PubMed] [Google Scholar]
- Uksila J., Lassila O., Hirvonen T., Toivanen P. Development of natural killer cell function in the human fetus. J Immunol. 1983 Jan;130(1):153–156. [PubMed] [Google Scholar]
- Vitiello A., Maccario R., Montagna D., Porta F. A., Alberini C. M., Mingrat G., Astaldi-Ricotti G. C., Nespoli L., Ugazio A. G. Lymphocyte subpopulations in the neonate: a subset of HNK-1-, OKT3-, OKT8+ lymphocytes displays natural killer activity. Cell Immunol. 1984 Apr 15;85(1):252–257. doi: 10.1016/0008-8749(84)90295-8. [DOI] [PubMed] [Google Scholar]
- Welte K., Andreeff M., Platzer E., Holloway K., Rubin B. Y., Moore M. A., Mertelsmann R. Interleukin 2 regulates the expression of Tac antigen on peripheral blood T lymphocytes. J Exp Med. 1984 Nov 1;160(5):1390–1403. doi: 10.1084/jem.160.5.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]


