Abstract
Overexpression of cAMP-dependent protein kinase (PKA) type I isozyme is associated with cell proliferation and neoplastic transformation. The presence of PKA on the external surface of LS-174T human colon carcinoma cells has been shown. Here, we show that cancer cells of various cell types excrete PKA into the conditioned medium. This extracellular PKA (ECPKA) is present in active, free catalytic subunit (C subunit) form, and its activity is specifically inhibited by PKA inhibitory protein, PKI. Overexpression of the Cα or RIα subunit gene of PKA in an expression vector, which up-regulates intracellular PKA type I, markedly up-regulates ECPKA expression. In contrast, overexpression of the RIIβ subunit, which eliminates PKA type I, up-regulates PKA type II, and reverts the transformed phenotype, down-regulates ECPKA. A mutation in the Cα gene that prevents myristylation allows the intracellular PKA up-regulation but blocks the ECPKA increase, suggesting that the NH2-terminal myristyl group of Cα is required for the ECPKA expression. In serum of cancer patients, the ECPKA expression is up-regulated 10-fold as compared with normal serum. These results indicate that the ECPKA expression is an ordered cellular response of a living cell to actively exclude excess intracellular PKA molecules from the cell. This phenomenon is up-regulated in tumor cells and has an inverse relationship with the hormone dependency of breast cancer. Thus, the extracellular PKA may serve as a potential diagnostic and prognostic marker for cancer.
In mammalian cells, there are two types of cAMP-dependent protein kinase (PKA) (1), type I (PKA-I) and type II (PKA-II), which are distinguished by different regulatory subunits (R subunits), RI and RII, as they share a common catalytic subunit (C subunit) (2). Through biochemical studies and gene cloning, four isoforms of the R subunits, RIα, RIβ, RIIα, and RIIβ, have been identified (3, 4) Importantly, the ratios of PKA-I to PKA-II can change dramatically during cell development, differentiation, and transformation (5, 6).
Increased expression of the RIα/PKA-I has been shown in human cancer cell lines and in primary tumors, as compared with normal counterparts (6, 7) in cells after transformation with chemical or viral carcinogens, the Ki-ras oncogene or transforming growth factor-α, and on stimulation of cell growth with granulocyte-macrophage colony-stimulating factor or phorbol esters (6). Conversely, a decrease in the expression of RIα/PKA-I correlates with growth inhibition induced by site-selective cAMP analogs in a broad spectrum of human cancer cell lines (8).
The presence of PKA on the external surface of LS-174T human colon carcinoma cells has recently been discovered (9). This ecto-PKA is immunologically and biochemically related to the intracellular soluble PKA. The ecto-PKA is stimulated by cAMP in phosphorylating a synthetic peptide substrate of PKA, Kemptide, and is specifically inhibited by PKA inhibitory protein, PKI (Walsh–Krebs inhibitor). The source of cAMP for activating the ecto-PKA comes from the intracellular source upon its secretion after forskolin treatment, and probenecid, which inhibits the secretion of cAMP (9), blocks the forskolin-mediated activation of ecto-PKA.
The cell surface serves as a key element in many cellular functions, signaling and cell communication, involving receptor molecules and ecto-enzymes. Cell surface components are thought to be modulated either by down-regulation, e.g., rapid turnover rate release of proteins from the surface or modification of proteins such as phosphorylation.
In the present study, we investigated whether PKA is released into the extracellular space and whether such release of PKA can reflect cell transformation, and consequently, can be a cancer biomarker. We also provide insights into the intracellular regulatory mechanism and structural requirement for extracellular PKA (ECPKA) expression by using a prostate cancer cell line; thus, this work goes beyond what has been done with the prostate-specific antigen.
Experimental Procedures
Cells and Cell Culture.
J82-T24 and UM-UC-3 bladder carcinoma cells and HCT-15 and LS-174T colon carcinoma cells were grown in MEM supplemented with 10% heat-inactivated FBS, 0.1 mM MEM nonessential amino acids (pH 7.4), and 1% antibiotic–antimycotic. CoLo205 colon carcinoma cells were grown in RPMI medium 1640 (GIBCO/BRL) supplemented with 10 mM Hepes (pH 7.4), 1 mM sodium pyruvate, 10% heat-inactivated FBS, and 1% antibiotic–antimycotic. MCF-7, MDA-MB-231, T-47D, SK-BR-3, and MCF-7TH breast carcinoma cells were grown in improved MEM (IMEM; Biofluids, Rockville, MD) supplemented with 10% heat-inactivated FBS and 1% antibiotic–antimycotic. 293 and 293T cells were grown in DMEM (Biofluids) supplemented with 10% heat-inactivated FBS and 1% antibiotic–antimycotic. A549 cells were grown in Ham's F-12 medium (Biofluids) supplemented with 10% heat-inactivated FBS, 0.1 mM MEM nonessential amino acids (pH 7.4), and 1% antibiotic–antimycotic. PC3M, PC3, DU145, and LNCap prostate carcinoma cells were grown in RPMI medium 1640 supplemented with 10% heat-inactivated FBS, 0.1 mM MEM nonessential amino acids (pH 7.4), and 1% antibiotic–antimycotic. PrEC5500 cells were grown in prostate epithelial cell basal medium (PrEBM) supplemented with prostate epithelial cell growth medium (PrEGM) (Clonetics, San Diego).
For PKA enzyme assays, cells were seeded at a density of 2–7 × 105 cells per 60-mm plate. When the cells were about 50–60% confluent, the culture medium was removed and 2 ml of fresh medium was added. After 24 hr of incubation, the conditioned medium was collected for PKA and lactate dehydrogenase (LDH) assays. Cells were harvested for cell count, and cell extracts were prepared as described in ref. 10 for intracellular PKA assay.
PKA Assays.
The enzyme activity of PKA was measured by the method described previously (11). For the measurement of conditioned medium PKA activity, the assays (total volume, 250 μl) were carried out for 20 min at 37°C in the reaction mixture containing 50 mM Tris⋅HCl (pH 7.5), 1 mM DTT, 10 mM MgCl2, 5 μM Kemptide (A Ser peptide; Leu-Arg-Arg-Ala-Ser-Leu-Gly; GIBCO/BRL), 1.2 μM [γ-32P]ATP (25 Ci/mmol; ICN) with or without 5 μM cAMP and 5 μM PKI, and 200 μl of conditioned medium. For cell extract PKA measurement, the assays (total volume, 50 μl) were carried out for 5 min at 30°C in the reaction mixture (see above) with 10 μg protein of cell extracts. After incubation, the reaction mixtures were spotted onto phosphocellulose disks (GIBCO/BRL) and were washed three times in 0.5% phosphoric acid. Filters were air-dried and then counted by liquid scintillation counter. One unit of PKA activity is defined as that amount of enzyme that transferred 1 pmol of 32P from [γ-32P]ATP to recovered protein in 1 min at 30o/37°C in the standard assay system. LDH activity was measured by the use of a commercial kit (Sigma). For cell count, cells were trypsinized and counted by using a ZI Coulter counter.
Mutagenesis of Cα and Construction of Retro-Viral Vector.
The Cα-Ala mutant gene was generated by subcloning a BamHI/SalI fragment containing the complete open frame of human Cα cDNA (12) into the vector pGEX-4T-1 (Amersham Pharmacia) and introducing two mutations into the gene (13), thereby altering the NH2-terminal Gly (GGC) to an Ala (GCA), by the use of the Site-Directed Mutagenesis System (Stratagene, cat. no. 200518). The following primers were used (mutation underlined): 5′-ccg-cgt-gga-tcc-atg-gca-aac-gcc-gcc-gcc-3′ and 5′-ggc-ggc-ggc-ggc-gtt-tgc-cat-gga-tcc-acg-cgg-3′. DNA sequencing analysis verified that no additional mutations were introduced. The BamHI/NotI of pGEX-Cα (wild type or mutant) vector fragment was inserted into the vector pcDNA3 (Invitrogen), and then the HindIII/XbaI fragment was inserted into the vector pGEM-11zf(+) (Promega). Finally, the pGEM-11zf(+) Cα vector was cut with BamHI, and the fragment was cloned into the BamHI site of the vector OT1529 (14) to produce the retroviral vector MT-1 (15).
Transfection and Production of Stable Lines.
PC3M cells (106 cells/100-mm plate) were transfected with 7.5 μg of MT-expression vector plasmid containing Cα, Cα mut, RIα, or RIIβ gene by the lipofectin method (GIBCO/BRL). The neomycin analog G418 (400 μg/ml) resistant colonies were isolated. Then the colonies were grown in the presence of 60 μM ZnSO4 (6 days), and clones overexpressing the transfected gene were pooled and used for the experiments.
DEAE-Sephacel Chromatography.
The cell pellets (4 × 107 cells) were washed two times with ice-cold isotonic PBS, suspended in 15 ml of 10 mM Tris⋅HCl buffer (pH 7.1) (containing 1 mM EDTA, 1 mM DTT, 0.5 mM PMSF, 1 mM benzamidine, 30 μg/ml leupeptin, 5.0 μg/ml aprotinin, and 5.0 μg/ml pepstatin), kept on ice for 30 min, homogenized (70 strokes) with Dounce homogenizer, centrifuged at 10,000 × g for 20 min, and filtered through a 0.45-μm pored syringe filter. The supernatants were used as the cell extracts for chromatography. Cell extracts (10 mg protein) were loaded onto DEAE columns (0.9 × 5.0 cm) and fractionated with a linear salt gradient (11). PKA assay (total volume, 100 μl) was carried out as described in Experimental Procedures (see PKA Assays) by using 50 μl of column fractions.
Western Blotting.
An amount of 10 ml of conditioned medium of PC3M cells was concentrated 150 times with microcon (Millipore). Then, 10 μg protein of cell extracts or 20 μl of concentrated conditioned medium were subjected to SDS/PAGE, and separated proteins were transferred to nitrocellulose membranes. Blots were blocked with 5% nonfat milk and 1% BSA for 1 hr at 4°C and probed with monoclonal antibodies to Cα or RIα (PharMingen; Transduction Laboratories, Lexington, KY) for 4 hr at 4°C. Blots were then washed and incubated with horseradish peroxidase-conjugated secondary antibodies and visualized by using the Amersham ECL System (Amersham Pharmacia).
Results and Discussion
cAMP-Dependent Protein Kinase in the Conditioned Medium of Cultured Cancer Cells.
We measured the PKA activity in the conditioned medium obtained from cancer cells grown in tissue culture for a given time. As the substrate for the PKA, we used Kemptide, which carries the specific recognition and phosphorylation sites for PKA. To measure the free C subunit activity and total PKA activity, the assays were performed in the absence and presence, respectively, of the added cAMP. To measure the PKA activity specifically without contamination by other kinases, we used the specific inhibitory protein for PKA, PKI (Walsh–Krebs inhibitor), and only the kinase activity, that is PKI-inhibitable, was taken as the PKA activity.
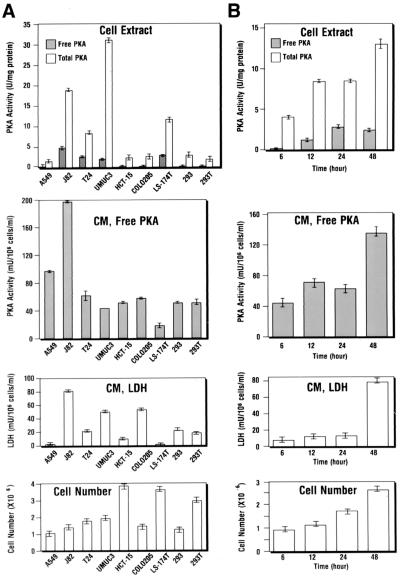
In parallel with the ECPKA assay of the conditioned medium, we also measured the intracellular PKA activity in the cell extracts prepared from the cells of the same culture from which the conditioned medium was obtained. As shown in Fig. 1A (Second Panel), varying degrees of ECPKA activity were detected in the conditioned medium from cancer cell lines of various cell types, including lung (A549), bladder (J82, T24, UMUC3), colon (HCT-15, CoLo205, LS-174T), and renal (293, 293T) carcinoma cells. The PKA activity detected in the conditioned medium was not activated by exogenously added cAMP (Fig. 1A, Second Panel). Thus, the PKA activity represents the free C subunit activity. This is in sharp contrast with the intracellular PKA activity in the cell extracts. There was almost no free C activity and only in the presence of exogenous cAMP was intracellular PKA detected (Fig. 1A Top), indicating that the intracellular PKA was exclusively present in an inactive holoenzyme form. Importantly, the pattern of ECPKA activity detected in conditioned medium of different cell lines did not correlate with the pattern of intracellular PKA activity, the conditioned medium–LDH activity, or the cell number in the culture dish (Fig. 1A). Thus, the ECPKA expression appears to be a unique characteristic of individual cancer cell lines and is unrelated to nonspecific cell damage-related phenomenon.
Figure 1.
Extracellular protein kinase A expression in the conditioned medium of cancer cells of various cell types. (A) ECPKA expression of lung (A549), bladder (J82, T24, UM-UC-3), colon (HCT-15, CoLo205, LS-174T), and renal (293, 293T) carcinoma cells. (B) Time course of ECPKA expression of T24 bladder carcinomas cells. ■, Free PKA, PKA activity measured in (−) cAMP; □, Total PKA, PKA activity measured in (+) cAMP; Background kinase activity, kinase activity measured in (±) cAMP and (+) PKI. The background activity was subtracted from both free PKA and total PKA; CM, conditioned medium; LDH, Lactate dehydrogenase. For cell extract PKA, both free and total PKA activities are shown in “U/mg protein”; For conditioned medium PKA, only free activity is shown in “mU/106 cells/ml” in 24 hr of culture that contained 2 ml of medium. Cell number, cell count at the end of cell culture (A) or at indicated times (B). Statistical analysis of difference between mean values was performed by the use of a two-tailed t test (statview statistical software; Abacus Concepts, Berkeley, CA).
We next examined the temporal accumulation of ECPKA in the conditioned medium of T24 bladder carcinoma cells. As shown in Fig. 1B (Second Panel), the ECPKA increased in the conditioned medium in a time-dependent manner. The kinase activity showed a peak activity at 12 hr of culture and plateaued thereafter up to 24 hr. At 48 hr, the PKA activity further increased, showing a biphasic curve of the activity. This pattern of the time-dependent increase of the ECPKA was similar to that of intracellular PKA (Fig. 1B Top) and the cell number increase (Fig. 1B Bottom). Thus, in a given cell, the ECPKA was expressed as a function of cell growth and intracellular PKA. Because the LDH activity can increase at 48 hr of culture (Fig. 1B Third Panel), ECPKA activity was measured at 24 hr of cell culture to avoid any nonspecific cell damage-related excretion of PKA.
Extracellular PKA Expression Is Inversely Related to Hormone-Dependency in Breast Cancer Cells.
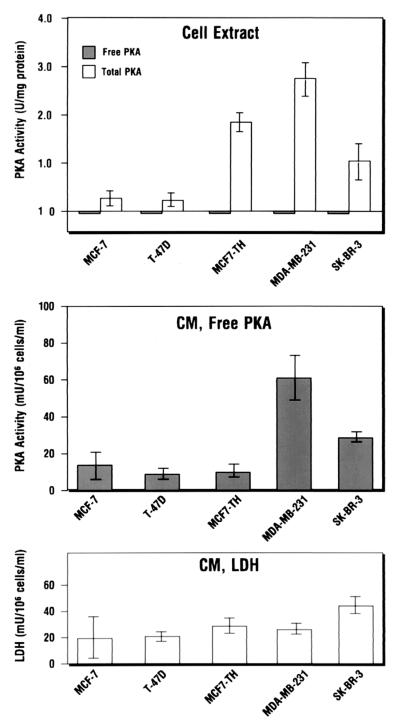
We have previously shown an inverse relationship between estrogen receptor and PKA during the growth and growth arrest of hormone-dependent breast cancer cells (16). We examined whether the expression of ECPKA is related to hormone dependency in breast cancer cells. The conditioned media from the 24-hr culture of hormone-dependent (MCF-7, T-47D), and -independent (MDA-MB-231, SK-BR-3), and hormone-dependent and multidrug-resistant (MCF-7TH) breast cancer cells were assayed for the ECPKA activity.
The ECPKA of these breast cancer cells was present in active, free C subunit form (Fig. 2 Middle), whereas the intracellular PKA was present in inactive holoenzyme form (Fig. 2 Top). The hormone-independent breast cancer cells expressed higher (5-fold) levels of ECPKA as compared with the hormone-dependent breast cancer cells (Fig. 2 Middle). This pattern of ECPKA expression paralleled intracellular PKA expression in these cells with the exception of MCF-7TH cells, which showed an inverse relation between the intracellular PKA and ECPKA (Fig. 2 Top and Middle). There was no correlation between LDH activity in the conditioned medium (Fig. 2 Bottom) and the ECPKA expression of these cells. These results support an inverse relationship between the ECPKA expression and hormone-dependency in breast cancer cells.
Figure 2.
Extracellular PKA expression is inversely related to hormone dependency of breast cancer cells. PKA activity was measured in cell extracts and conditioned medium of hormone-dependent (MCF-7, T-47D, MCF-7TH) and -independent (SK-BR-3, MDA-MB-231) human breast carcinoma cells by the method described in Experimental Procedures. Statistical analysis was performed by the method described in the Fig. 1 legend. Data represent mean values ± SD of three separate experiments.
Extracellular PKA expression Is Independent of Prostate-Specific Antigen (PSA) Expression.
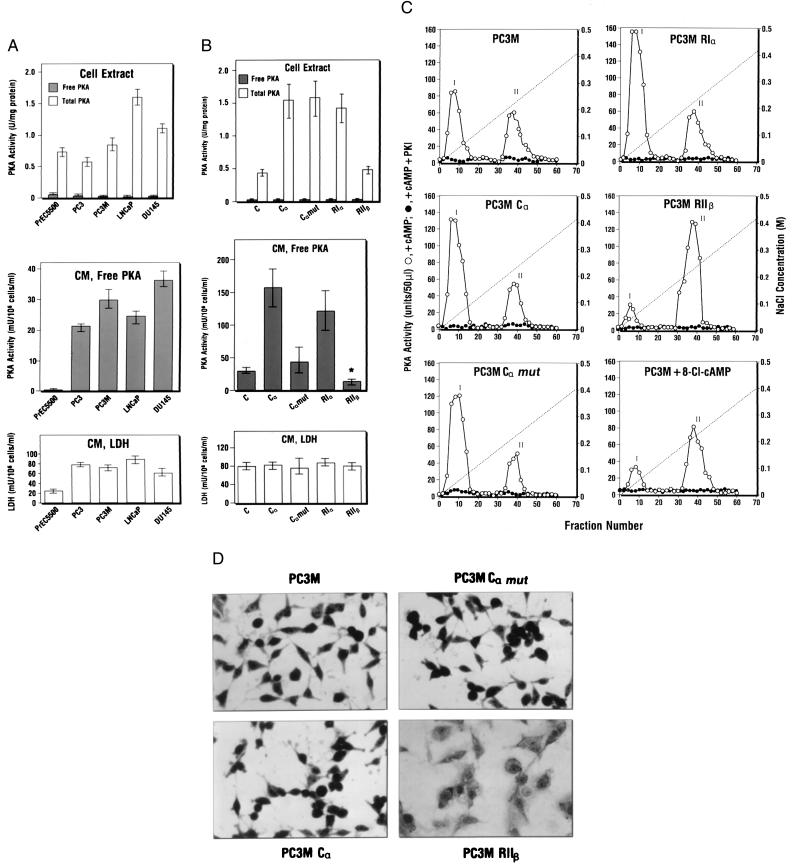
PSA determination has been used for diagnosis of prostate cancer (17). We examined the ECPKA expression in prostate cancer cells that express low and high levels of PSA. As shown in Fig 3A (Middle), the ECPKA levels in the conditioned medium of four different prostate cancer cell lines were 100- to180-fold greater than that of immortalized prostate epithelial cell line, PrEC5500. The amounts of PSA secreted by DU145 and LNCaP cells are 1.7 pg and 232 pg, respectively, per 106 cells per ml in 24 hr (18). Thus, these prostate cancer cells exhibited a high level of ECPKA regardless of their PSA levels. Importantly, the immortalized PrEC5500 cells contained the intracellular PKA at the level comparable to that in prostate cancer cells (Fig. 3A Top), but exhibited very low level (0.2 milliunit/106 cells/ml/24 hr) of ECPKA (Fig. 3A Middle). The patterns of ECPKA expression of these prostate cancer cell lines were distinct from their intracellular PKA expression patterns and were unrelated to the LDH expression (Fig. 3A Bottom).
Figure 3.
Extracellular PKA expression in prostate cancer cells is unrelated to PSA expression and is regulated by intracellular PKA. (A) PKA activity in cell extracts and conditioned medium of prostate cancer cells (PC3, PC3M, LNCap, DU145) and immortalized prostate epithelial cells (PrEC5500). (B) Effect of R and C subunit gene overexpression on intracellular PKA and ECPKA. C, Nontransfected parental cells; Cα, Cα mut, RIα, and RIIβ cells transfected with Cα, Cα mut, RIα, or RIIβ gene in retroviral vector MT-1 (see Experimental Procedures), respectively. PKA and LDH assays were performed as described in Experimental Procedures. Statistical analyses were carried out as described in the legend to Fig. 1. Data in A and B represent mean values ± SD obtained from three separate experiments (*, P < 0.05, vs. that of parental nontransfectant, C). (C) DEAE-column profile of PKA in cells overexpressing PKA subunit genes and cells treated with 8-Cl-cAMP (5 μM for 3 days). PC3M, Parental nontransfectant. For transfectants, see B. Cell extract preparation and DEAE-column chromatography were performed as described in Experimental Procedures. Peak I, PKA-I; Peak II, PKA-II. (D) Effect of PKA subunit overexpression on cell morphology. To examine whole-cell morphology, cells were washed with PBS, fixed with 70% methanol for 5 min, and stained with Giemsa (Sigma) for 15 min. After staining, the whole cells were visualized under an inverted microscope. (Magnification, ×320.)
PKA Isozyme-Specific Regulation of Extracellular PKA.
PKA isozyme type I (PKA-I), as opposed to type II isozyme (PKA-II), has been implicated in cell transformation (6). In untransformed NIH 3T3 cells, only type II PKA is present, and a sharp increase in type I PKA coincides with viral (SV40) transformation of these fibroblasts (19). Conversely, ras-transformed NIH 3T3 cells can be reverted to nontransformed phenotype by overexpression of the RIIβ subunit of PKA, which down-regulates type I PKA and up-regulates type II PKA (20). We examined whether PKA isozyme distribution in the cell contributes to ECPKA expression.
Alterations in PKA isozyme distribution in cancer cells can be brought about by overexpression of the R and C subunit genes of PKA (15, 10). PC3M cells transfected with Cα, Cα mutant, RIα, and RIIβ genes (see Experimental Procedures) were used to examine the intracellular PKA and ECPKA levels (Fig. 3B).
Cells overexpressing Cα exhibited a 3.5-fold increase in intracellular PKA and a 6-fold increase in ECPKA (Fig. 3B Top and Second Panels). RIα overexpression led to a 3-fold increase in the intracellular PKA and a 5-fold increase in ECPKA (Fig. 3B Top and Second Panels). This increase in ECPKA was not due to cell damage; LDH levels in the conditioned medium of transfectants remained the same as that in the parental cells (Fig. 3B Bottom). DEAE-column chromatography analysis showed that both Cα and RIα overexpression led to a marked increase in PKA-I holoenzyme level without affecting PKA-II level (Fig. 3C Middle Left and Top Right).
In contrast, the RIIβ overexpression led to no change in intracellular PKA level and, markedly, a significant reduction (P < 0.05) in the ECPKA expression (Fig. 3B Top and Middle). DEAE-column chromatography showed that RIIβ overexpression almost completely eliminated PKA-I and sharply up-regulated PKA-II (Fig. 3C Middle Right). Importantly, overexpression of RIIβ changed the cell morphology to that typical of flat phenotype (Fig. 3D) and retarded cell growth. Cα or RIα overexpressing cells exhibited no change in cell morphology (Fig. 3D) or cell growth.
Site-selective cAMP analogs can differentially regulate PKA isozymes (21, 22). Treatment of PC3M cells with a site-selective cAMP analog, 8-Cl-cAMP (5 μM for 3 days), led to down-regulation of both intracellular PKA and ECPKA and induced growth inhibition (data not shown). DEAE-column chromatography showed that these effects of 8-Cl-cAMP on the PKA inhibition and growth inhibition were clearly reflected in the selective down-regulation of PKA-I isozyme (Fig. 3C Bottom Right) (6, 11). These results indicate that up-regulation of ECPKA may correlate with neoplastic cell transformation in which intracellular PKA-I is increased.
A Myristate-Lacking Mutant of Cα Is Incapable of Excreting into Extracellular Space.
The Cα subunit of PKA is acylated at its NH2 terminus with myristic acid (23). This type of modification is thought to mediate the association of proteins with lipid bilayers, yet the C subunit shows no preferential binding with membranes (24). In the C subunit of sperm, the amino-terminal myristate and the first 14 amino acids of Cα are replaced by an amino-terminal acetate and 6 different amino acids (25). It has been suggested that this different amino terminus of Cα may be related to a unique requirement for localization of the free C subunit within the sperm flagellum (25).
We investigated the possible role of C subunit myristylation with respect to ECPKA expression by using cDNA expression vector OT1529 (14), in which the acylated NH2-terminal Gly of Cα was mutated to Ala. As shown in Fig. 3B (Top), the mutant Cα, Cα-Ala overexpressing cells sharply increased the intracellular PKA levels to the same extent as the wild-type Cα overexpressing cells. However, unlike the wild-type Cα overexpressing cells, which markedly increased the ECPKA level, the mutant Cα-Ala overexpressing cells were unable to increase the ECPKA level above that of nontransfected parental cells (Fig. 3B Middle). DEAE-column chromatography analysis showed that the mutant Cα-Ala cells were capable of inducing PKA-I holoenzyme to the same extent as the wild-type Cα cells (Fig. 3C Middle Left and Bottom Left). These results indicate that the N-terminal myristylation is an essential requirement for the C subunit excretion into the extracellular space.
Extracellular PKA Is Immunologically and Biochemically Identical to Intracellular PKA.
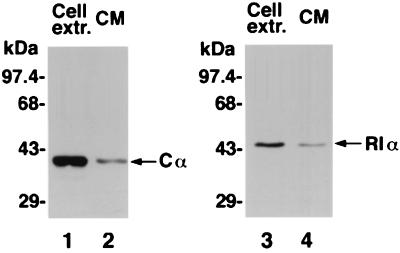
We examined whether the ECPKA identified in the present study is identical to the intracellular PKA. Cell extracts and 150-fold concentrated conditioned medium prepared from PC3M prostate cancer cells were subjected to Western blot analysis. Probing with anti-human Cα antibody identified the presence of Cα protein in both cell extract and conditioned medium. The Cα protein from cell extract and conditioned medium comigrated the same distance in SDS/PAGE exhibiting a single protein band of 40 kDa (Fig. 4, lanes 1 and 2). When probed with anti-human RIα antibody, a single protein band of 48 kDa RIα was detected in both cell extract and conditioned medium (Fig. 4, lanes 3 and 4). RIIα and RIIβ proteins were detected only in the cell extract but not in the conditioned medium (data not shown). These results indicate that the ECPKA may be a type I PKA.
Figure 4.
Immunological characterization of extracellular PKA. Lanes 1 and 2, Western blotting of cell extract and conditioned medium (CM) with anti-Cα antibody. Lanes 3 and 4, Western blotting of cell extract and CM with anti-RIα antibody.
The catalytic properties of ECPKA were examined in comparison to that of intracellular PKA (data not shown). The ECPKA and intracellular PKA activities were proportional to incubation time for at least 30 min and 5 min, respectively, and were proportional to enzyme concentration up to at least 300 μl of conditioned medium and 40 μg of protein, respectively. The apparent Michaelis constant (Km) of the ECPKA for Kemptide (4.0 μM) is similar to the apparent Km of intracellular PKA for Kemptide (4.5 μM), and PKI at 10 μM inhibited the intracellular PKA and ECPKA activities, 90% and 50%, respectively. cAMP at varying concentrations markedly stimulated the intracellular PKA but not the ECPKA. These results indicate that the ECPKA and intracellular PKA are similar in their catalytic properties but are distinct in their enzyme form; the former is present in active, free C subunit form, and the latter is present in inactive holoenzyme form.
Extracellular PKA in Serum of Cancer Patients.
We next examined the ECPKA expression of human tumors. A total of 100 serum samples examined were from patients with variety of cancers, including renal, colon, rectal, adrenal and lung carcinomas, melanomas, and lymphomas. A total of 14 serum samples from normal people were used as control.
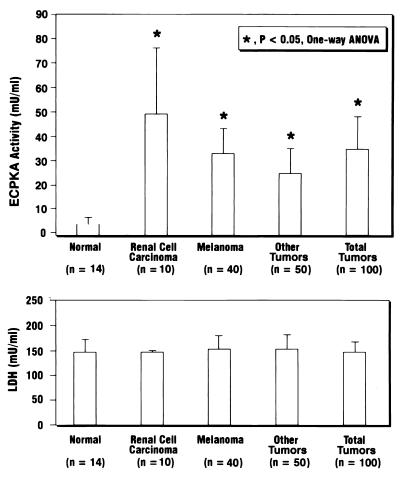
Fig. 5 shows that the ECPKA activity was significantly elevated (P < 0.05) in the serum samples of cancer patients as compared with that in normal serum samples. The mean ± SD values of PKA activity (milliunits/ml) in the sera of renal cell carcinoma, melanoma, and other cancer patients were 49.2 ± 25.0 (range, 26.5 to 113.0; n = 10), 32.0 ± 10.5 (range: 15.4 to 73.3; n = 40), and 25.5 ± 10.0 (range, 15.0 to 62.4; n = 50), respectively. Thus, the average ECPKA level in the patients of all cancer (mean ± SD = 35.0 ± 12.5; n = 100) was 10-fold higher (P < 0.05) than the average PKA value of normal human serum (mean ± SD = 2.70 ± 0.80; range, 0 to 10.6, n = 14) (Fig. 5).
Figure 5.
Detection of ECPKA in the serum of cancer patients. Human serum samples were from renal cell carcinoma patients (n = 10); melanoma patients (n = 40); other cancer patients with colon, rectal, adrenal, and lung carcinomas and lymphomas (n = 50); and normal people (n = 14). The statistical analysis of the data was performed by the use of one-way ANOVA (*, P < 0.05). The PKA assay was carried out by the method described in Experimental Procedures with 10 μl of serum samples in the assay (total volume, 50 μl). LDH assay was carried out as described in Experimental Procedures with 10 μl of 6-fold-diluted serum.
The LDH levels of all serum samples were within comparable values of 148–158 milliunits/ml (normal range, 55–170 milliunits/ml) (Fig. 5). This indicates that no appreciable cell degradation accounted for the ECPKA detected in the human serum samples. The ECPKA detected in the human sera was not stimulated with cAMP, but was inhibited by the PKA inhibitor, PKI. This indicates that the ECPKA in the human sera was present in active, free C subunit form.
Conclusions
We have shown in the present study that a living cell can excrete the free C subunit of PKA into the extracellular space. Biochemical and immunological characterization showed that this extracellular PKA is a type I PKA. Given the enhanced sensitivity to cAMP activation of type I vs. type II PKA holoenzyme (26, 27), this ECPKA expression may be an important mechanism for regulating the cellular PKA type I/type II holoenzyme ratio, and for maintaining regulation of C subunit levels in the cell. Type I PKA is excessively expressed in tumor cells (6, 7), and our findings show that the ECPKA expression is increased in the conditioned medium of cancer cells and serum samples of cancer patients. Importantly, this ECPKA up-regulation is reduced in cancer cells maintaining hormone-dependency (a normal cell property), such as hormone-dependent breast cancer, in cancer cells of reverted phenotype (RIIβ subunit overexpression), and in cells overexpressing Cα-Ala mutant lacking myristic acid. Taken together, our results show evidence that cAMP regulation of PKA in mammalian cells can occur in the extracellular space, and this phenomenon may provide an innovative approach to cancer diagnosis and therapy.
Acknowledgments
We thank John W. Greiner for providing us with LS-174T cells, Kenneth H. Cowan for 293 cells, David Salomon for T-47D and SK-BR-3 cells, Kwong Y. Tsang for PC3 and DU145 cells, C. A. Stein for LNCap cells, S. Hanks for human Cα cDNA, and M. L. McGeady for OT1521 and OT1529 vectors.
Abbreviations
- PKA
cAMP-dependent protein kinase
- C subunit
catalytic subunit
- R subunit
regulatory subunit
- PKI
protein kinase inhibitor protein
- ECPKA
extracellular PKA
- LDH
lactate dehydrogenase
- PSA
prostate-specific antigen
References
- 1.Krebs E G, Beavo J A. Annu Rev Biochem. 1979;48:923–939. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- 2.Beebe S J, Corbin J D. The Enzymes: Control by Phosphorylation. Vol. 17. New York: Academic; 1986. pp. 43–111. [Google Scholar]
- 3.McKnight G S, Clegg C H, Uhler M D, Chrivia J C, Cadd G G, Correll L A, Otten A D. Recent Prog Horm Res. 1988;44:307–335. doi: 10.1016/b978-0-12-571144-9.50014-4. [DOI] [PubMed] [Google Scholar]
- 4.Levy F O, Oyen O, Sandberg M, Tasken K, Eskild W, Hansson V, Jahnsen T. Mol Endocrinol. 1988;2:1364–1373. doi: 10.1210/mend-2-12-1364. [DOI] [PubMed] [Google Scholar]
- 5.Lohmann S M, Walter U. In: Advances in Cyclic Nucleotide and Protein Phosphorylation Research. Greengard P, Robison G A, editors. Vol. 18. New York: Raven; 1984. pp. 63–117. [PubMed] [Google Scholar]
- 6.Cho-Chung Y S. Cancer Res. 1990;50:7093–7100. [PubMed] [Google Scholar]
- 7.Miller W R, Hulme M J, Cho-Chung Y S, Elton R A. Eur J Cancer. 1993;29A:989–991. doi: 10.1016/s0959-8049(05)80207-2. [DOI] [PubMed] [Google Scholar]
- 8.Cho-Chung Y S, Clair T, Tagliaferri P, Ally S, Katsaros D, Tortora G, Neckers L, Avery T L, Crabtree G W, Robins R K. Cancer Invest. 1989;7:161–177. doi: 10.3109/07357908909038282. [DOI] [PubMed] [Google Scholar]
- 9.Kondrashin A, Nesterova M, Cho-Chung Y S. Biochemistry. 1999;38:172–179. doi: 10.1021/bi982090e. [DOI] [PubMed] [Google Scholar]
- 10.Nesterova M, Yokozaki H, McDuffie E, Cho-Chung Y S. Eur J Biochem. 1996;235:486–494. doi: 10.1111/j.1432-1033.1996.00486.x. [DOI] [PubMed] [Google Scholar]
- 11.Rohlff C, Clair T, Cho-Chung Y S. J Biol Chem. 1993;268:5774–5782. [PubMed] [Google Scholar]
- 12.Maldonado F, Hanks S K. Nucleic Acids Res. 1988;16:8189–8190. doi: 10.1093/nar/16.16.8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamps M P, Buss J E, Sefton B M. Cell. 1988;46:105–112. doi: 10.1016/0092-8674(86)90542-8. [DOI] [PubMed] [Google Scholar]
- 14.McGeady M L, Kerby S S, Shankar V, Ciardiello F, Salomon D S, Seidman M. Oncogene. 1989;4:1375–1382. [PubMed] [Google Scholar]
- 15.Tortora G, Cho-Chung Y S. J Biol Chem. 1990;265:18067–18070. [PubMed] [Google Scholar]
- 16.Cho-Chung Y S, Clair T, Bodwin J S, Berghoffer B. Science. 1981;214:77–79. doi: 10.1126/science.6269181. [DOI] [PubMed] [Google Scholar]
- 17.Wirth M, Manseck A, Heimbach D. Eur Urol. 1993;24, Suppl. 2:6–12. doi: 10.1159/000474380. [DOI] [PubMed] [Google Scholar]
- 18.Correale P, Walmsley K, Nieroda C, Zaremba S, Zhu M, Schlom J, Tsang K Y. J Natl Cancer Inst. 1997;89:293–300. doi: 10.1093/jnci/89.4.293. [DOI] [PubMed] [Google Scholar]
- 19.Gharrett A J, Malkinson A M, Sheppard J R. Nature (London) 1976;264:673–675. doi: 10.1038/264673a0. [DOI] [PubMed] [Google Scholar]
- 20.Budillon A, Cereseto A, Kondrashin A, Nesterova M, Merlo G, Clair T, Cho-Chung Y S. Proc Natl Acad Sci USA. 1995;92:10634–10638. doi: 10.1073/pnas.92.23.10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doskeland S O. Biochem Biophys Res Commun. 1978;83:543–549. doi: 10.1016/0006-291x(78)91024-0. [DOI] [PubMed] [Google Scholar]
- 22.Rannels S R, Corbin J D. J Biol Chem. 1980;255:7085–7088. [PubMed] [Google Scholar]
- 23.Carr S A, Biemann K, Shoji S, Parmelee D C, Titani K. Proc Natl Acad Sci USA. 1982;79:6128–6131. doi: 10.1073/pnas.79.20.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nigg E A, Schafer G, Hilz H, Eppenberger H M. Cell. 1985;41:1039–1051. doi: 10.1016/s0092-8674(85)80084-2. [DOI] [PubMed] [Google Scholar]
- 25.San Agustin J E, Leszyk J D, Nuwaysir L M, Witman G B. J Biol Chem. 1998;38:24874–24888. doi: 10.1074/jbc.273.38.24874. [DOI] [PubMed] [Google Scholar]
- 26.Cummings D E, Brandon E P, Planas J V, Motamed K, Idzerda R L, McKnight S. Nature (London) 1998;382:622–626. doi: 10.1038/382622a0. [DOI] [PubMed] [Google Scholar]
- 27.Lee G R, Kim S N, Noguchi K, Park S D, Hong S H, Cho-Chung Y S. Mol Cell Biochem. 1999;195:77–86. doi: 10.1023/a:1006934113439. [DOI] [PubMed] [Google Scholar]