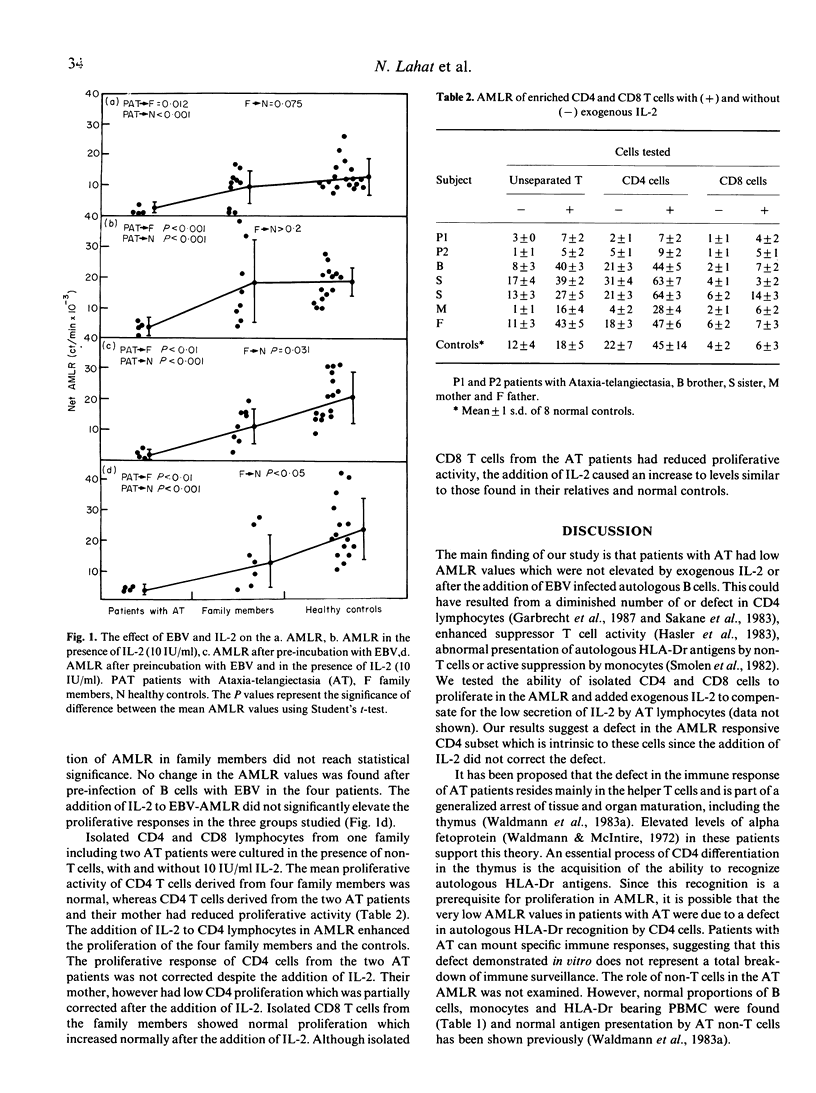
Abstract
We used the autologous mixed lymphocyte reaction (AMLR) to test T cell function in four patients with Ataxia-telangiectasia (AT), in 11 first-degree relatives and in 20 controls. There was a marked reduction of AMLR in the patients and in three relatives compared to the age-matched controls. In the AT patients the defect in AMLR was intrinsic to the CD4 subpopulation, since exogenous IL-2 did not improve the response of isolated CD4 cells. In contrast to normal controls, pre-incubation of autologous B cells with Epstein-Barr virus (EBV) did not enhance the reduced AMLR in the AT patients and the three first-degree relatives. We conclude that in both patients with AT and in some of their family members there is an intrinsic defect in CD4 T cells. This defect leads to diminished reactivity to EBV infected autologous B cells, and may explain in part the high incidence of malignancies observed in such families.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariga T., Sakiyama Y., Matsumoto S., Okano M., Osato T. Immunoglobulin production in Epstein-Barr virus-transformed lymphoblastoid cell lines in patients with ataxia telangiectasia. Int Arch Allergy Appl Immunol. 1985;78(1):33–36. doi: 10.1159/000233859. [DOI] [PubMed] [Google Scholar]
- Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 2-1987. A 30-year-old man with ataxia-telangiectasia and dysphagia. N Engl J Med. 1987 Jan 8;316(2):91–100. doi: 10.1056/NEJM198701083160206. [DOI] [PubMed] [Google Scholar]
- Etzioni A., Benderly A., Rosenthal E., Shehadah V., Auslander L., Lahat N., Pollack S. Defective humoral and cellular immune functions associated with veno-occlusive disease of the liver. J Pediatr. 1987 Apr;110(4):549–554. doi: 10.1016/s0022-3476(87)80546-2. [DOI] [PubMed] [Google Scholar]
- Garbrecht F. C., Siskind G. W., Weksler M. E. Lymphocyte transformation induced by autologous cells: XVIII. Impaired autologous mixed lymphocyte reaction in subjects with AIDS-related complex. Clin Exp Immunol. 1987 Feb;67(2):245–251. [PMC free article] [PubMed] [Google Scholar]
- Hasler F., Bluestein H. G., Zvaifler N. J., Epstein L. B. Analysis of the defects responsible for the impaired regulation of EBV-induced B cell proliferation by rheumatoid arthritis lymphocytes. II. Role of monocytes and the increased sensitivity of rheumatoid arthritis lymphocytes to prostaglandin E. J Immunol. 1983 Aug;131(2):768–772. [PubMed] [Google Scholar]
- James S. P., Yenokida G. G., Graeff A. S., Elson C. O., Strober W. Immunoregulatory function of T cells activated in the autologous mixed lymphocyte reaction. J Immunol. 1981 Dec;127(6):2605–2609. [PubMed] [Google Scholar]
- Klein E., Masucci M. G. Cell-mediated immunity against Epstein-Barr virus infected B lymphocytes. Springer Semin Immunopathol. 1982;5(1):63–73. doi: 10.1007/BF00201957. [DOI] [PubMed] [Google Scholar]
- Masucci G., Berkel I., Masucci M. G., Ernberg I., Szigeti R., Ersoy F., Sanal O., Yegin O., Henle G., Henle W. Epstein-Barr virus (EBV)-specific cell-mediated and humoral immune responses in ataxia-telangectasia patients. J Clin Immunol. 1984 Sep;4(5):369–382. doi: 10.1007/BF00917140. [DOI] [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSON R. D., KELLY W. D., GOOD R. A. ATAXIA-TELANGIECTASIA. ITS ASSOCIATION WITH A DEFECTIVE THYMUS, IMMUNOLOGICAL-DEFICIENCY DISEASE, AND MALIGNANCY. Lancet. 1964 May 30;1(7344):1189–1193. doi: 10.1016/s0140-6736(64)91209-7. [DOI] [PubMed] [Google Scholar]
- Paterson M. C., Smith B. P., Lohman P. H., Anderson A. K., Fishman L. Defective excision repair of gamma-ray-damaged DNA in human (ataxia telangiectasia) fibroblasts. Nature. 1976 Apr 1;260(5550):444–447. doi: 10.1038/260444a0. [DOI] [PubMed] [Google Scholar]
- Pellegrino M. A., Ferrone S., Dierich M. P., Reisfeld R. A. Enhancement of sheep red blood cell human lymphocyte rosette formation by the sulfhydryl compound 2-amino ethylisothiouronium bromide. Clin Immunol Immunopathol. 1975 Jan;3(3):324–333. doi: 10.1016/0090-1229(75)90019-7. [DOI] [PubMed] [Google Scholar]
- Sakane T., Kotani H., Takada S., Murakawa Y., Ueda Y. A defect in the suppressor circuits among OKT4+ cell populations in patients with systemic lupus erythematosus occurs independently of a defect in the OKT8+ suppressor T cell function. J Immunol. 1983 Aug;131(2):753–761. [PubMed] [Google Scholar]
- Smolen J. S., Chused T. M., Novotny E. A., Steinberg A. D. The human autologous mixed lymphocyte reaction. III. Immune circuits. J Immunol. 1982 Sep;129(3):1050–1053. [PubMed] [Google Scholar]
- Swift M., Sholman L., Perry M., Chase C. Malignant neoplasms in the families of patients with ataxia-telangiectasia. Cancer Res. 1976 Jan;36(1):209–215. [PubMed] [Google Scholar]
- Thomas Y., Rogozinski L., Irigoyen O. H., Friedman S. M., Kung P. C., Goldstein G., Chess L. Functional analysis of human T cell subsets defined by monoclonal antibodies. IV. Induction of suppressor cells within the OKT4+ population. J Exp Med. 1981 Aug 1;154(2):459–467. doi: 10.1084/jem.154.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A., Broder S., Goldman C. K., Frost K., Korsmeyer S. J., Medici M. A. Disorders of B cells and helper T cells in the pathogenesis of the immunoglobulin deficiency of patients with ataxia telangiectasia. J Clin Invest. 1983 Feb;71(2):282–295. doi: 10.1172/JCI110768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A., McIntire K. R. Serum-alpha-fetoprotein levels in patients with ataxia-telangiectasia. Lancet. 1972 Nov 25;2(7787):1112–1115. doi: 10.1016/s0140-6736(72)92717-1. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A., Misiti J., Nelson D. L., Kraemer K. H. Ataxia-telangiectasis: a multisystem hereditary disease with immunodeficiency, impaired organ maturation, x-ray hypersensitivity, and a high incidence of neoplasia. Ann Intern Med. 1983 Sep;99(3):367–379. doi: 10.7326/0003-4819-99-3-367. [DOI] [PubMed] [Google Scholar]


