Abstract
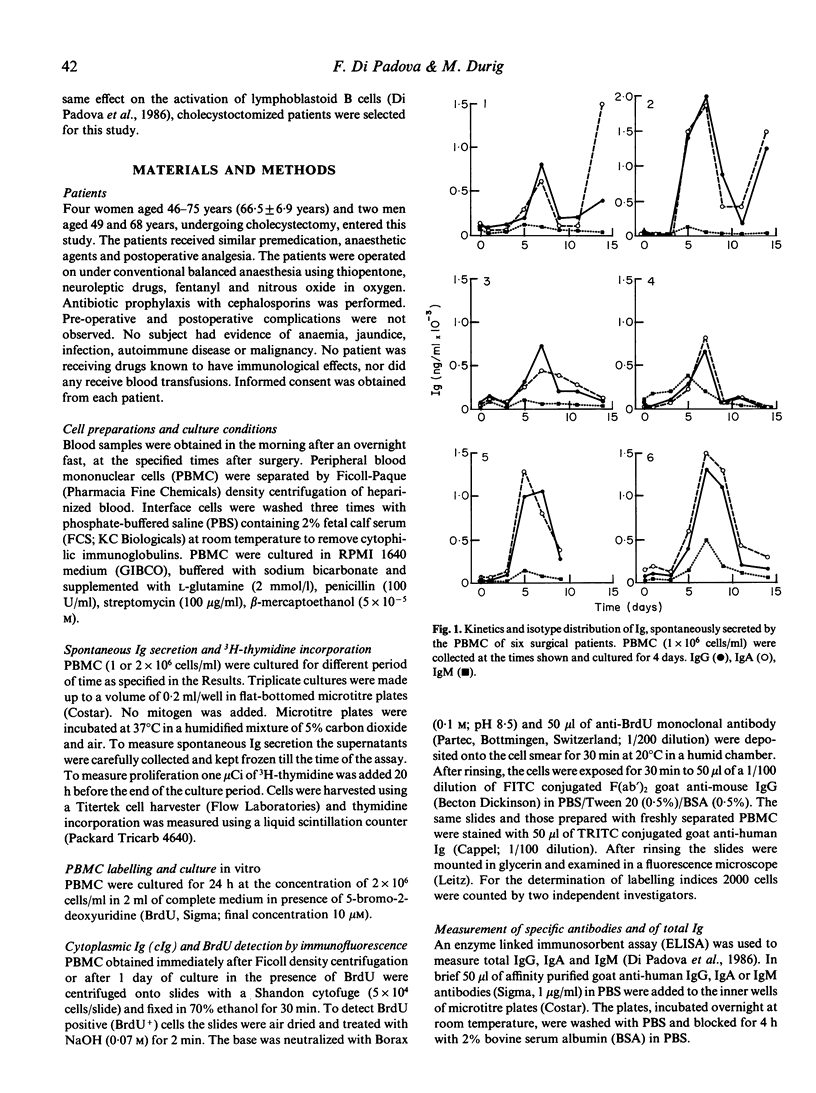
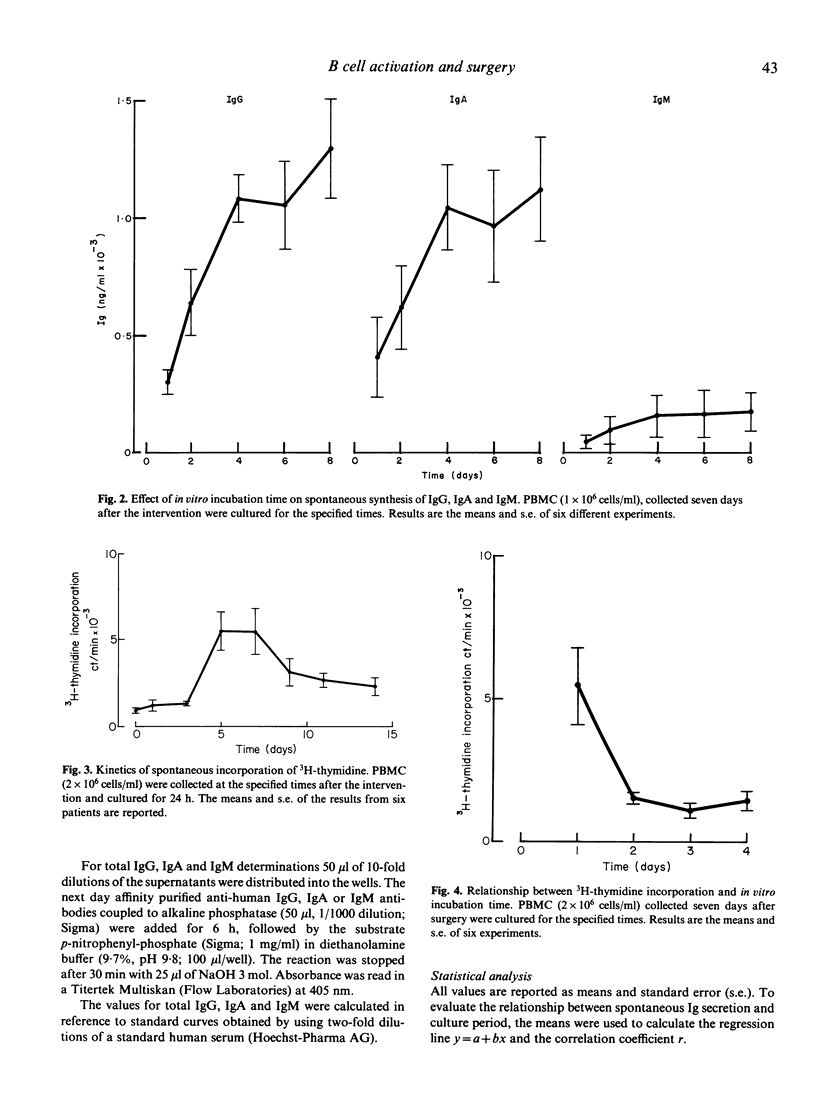
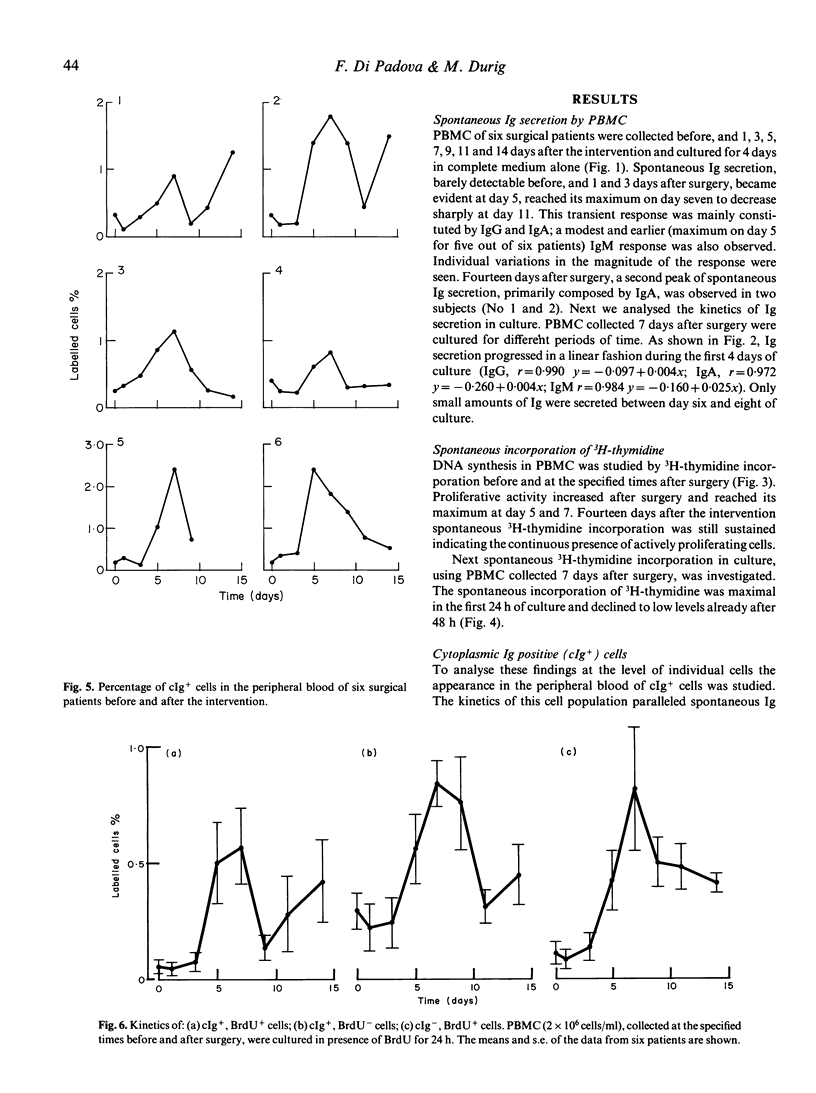
A state of immunosuppression or immunodeficiency has been described after surgical trauma. Cellular immune functions are more heavily affected. At present the relationship between these abnormalities and the increased incidence of infections in surgical patients has not been clarified. The activation of the humoral compartment and the appearance of lymphoblastoid B cells spontaneously secreting IgG and IgA have been observed in surgical patients. These cells are a marker of a recent antigenic exposure. In this study the kinetics of appearance of this B cell subset and the relationship between spontaneous Ig secretion and DNA synthesis have been analysed in six cholecystectomized patients. A peak of spontaneous IgG and IgA secretion is evident 5 and 7 days after the intervention. In some patients (two out of six) the appearance of lymphoblastoid B cells is cyclical. A second wave of spontaneous Ig secretion becomes evident 14 days after surgery. Double immunofluorescent staining of peripheral blood lymphocytes for BrdU and cytoplasmic Ig (cIg) was employed to demonstrate that a fraction of lymphoblastoid B cells is actively proliferating and that other cells negative for cIg but active in DNA synthesis appear in the circulation. These data confirm the signs of activation observed after elective surgery in otherwise healthy subjects.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyoshi T., Koba F., Arinaga S., Miyazaki S., Wada T., Tsuji H. Impaired production of interleukin-2 after surgery. Clin Exp Immunol. 1985 Jan;59(1):45–49. [PMC free article] [PubMed] [Google Scholar]
- Constantian M. B., Menzoian J. O., Nimberg R. B., Schmid K., Mannick J. A. Association of a circulating immunosuppressive polypeptide with operative and accidental trauma. Ann Surg. 1977 Jan;185(1):73–79. doi: 10.1097/00000658-197701000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerkinsky C., Prince S. J., Michalek S. M., Jackson S., Russell M. W., Moldoveanu Z., McGhee J. R., Mestecky J. IgA antibody-producing cells in peripheral blood after antigen ingestion: evidence for a common mucosal immune system in humans. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2449–2453. doi: 10.1073/pnas.84.8.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Padova F., Di Padova C., Rovagnati P., Tritapepe R. Appearance of spontaneously Ig secreting B cells in human peripheral blood after surgery. Clin Exp Immunol. 1986 Sep;65(3):582–588. [PMC free article] [PubMed] [Google Scholar]
- Di Padova F., Durig M., Di Padova C., Pozzoli M., Tritapepe R. Spontaneous and polyclonal Ig secretion by circulating B cells after surgery. Surgery. 1988 May;103(5):547–552. [PubMed] [Google Scholar]
- Di Padova F., Dürig M., Wadström J., Harder F. Role of spleen in immune response to polyvalent pneumococcal vaccine. Br Med J (Clin Res Ed) 1983 Dec 17;287(6408):1829–1832. doi: 10.1136/bmj.287.6408.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzelak I., Olszewski W. L., Engeset A. Suppressor cell activity in peripheral blood in cancer patients after surgery. Clin Exp Immunol. 1983 Jan;51(1):149–156. [PMC free article] [PubMed] [Google Scholar]
- Hamid J., Bancewicz J., Brown R., Ward C., Irving M. H., Ford W. L. The significance of changes in blood lymphocyte populations following surgical operations. Clin Exp Immunol. 1984 Apr;56(1):49–57. [PMC free article] [PubMed] [Google Scholar]
- Lennard T. W., Shenton B. K., Borzotta A., Donnelly P. K., White M., Gerrie L. M., Proud G., Taylor R. M. The influence of surgical operations on components of the human immune system. Br J Surg. 1985 Oct;72(10):771–776. doi: 10.1002/bjs.1800721002. [DOI] [PubMed] [Google Scholar]
- Lycke N., Lindholm L., Holmgren J. Cholera antibody production in vitro by peripheral blood lymphocytes following oral immunization of humans and mice. Clin Exp Immunol. 1985 Oct;62(1):39–47. [PMC free article] [PubMed] [Google Scholar]
- Meakins J. L., Christou N. V., Shizgal H. M., MacLean L. D. Therapeutic approaches to anergy in surgical patients. Surgery and levamisole. Ann Surg. 1979 Sep;190(3):286–296. doi: 10.1097/00000658-197909000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohr C. W., Christou N. V., Rode H., Gordon J., Meakins J. L. In vivo and in vitro humoral immunity in surgical patients. Ann Surg. 1984 Sep;200(3):373–380. doi: 10.1097/00000658-198409000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohr C. W., Latter D. A., Meakins J. L., Christou N. V. In vivo and in vitro humoral immunity in surgical patients: antibody response to pneumococcal polysaccharide. Surgery. 1986 Aug;100(2):229–238. [PubMed] [Google Scholar]
- O'Mahony J. B., Wood J. J., Rodrick M. L., Mannick J. A. Changes in T lymphocyte subsets following injury. Assessment by flow cytometry and relationship to sepsis. Ann Surg. 1985 Nov;202(5):580–586. doi: 10.1097/00000658-198511000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oladimeji M., Grimshaw A. D., Baum M., Patterson K. G., Goldstone A. H. Effect of surgery on monocyte function. Br J Surg. 1982 Mar;69(3):145–146. doi: 10.1002/bjs.1800690309. [DOI] [PubMed] [Google Scholar]
- Rodrick M. L., Wood J. J., O'Mahony J. B., Davis C. F., Grbic J. T., Demling R. H., Moss N. M., Saporoschetz I., Jordan A., D'Eon P. Mechanisms of immunosuppression associated with severe nonthermal traumatic injuries in man: production of interleukin 1 and 2. J Clin Immunol. 1986 Jul;6(4):310–318. doi: 10.1007/BF00917332. [DOI] [PubMed] [Google Scholar]
- Romball C. G., Weigle W. O. A cyclical appearance of antibody-producing cells after a single injection of serum protein antigen. J Exp Med. 1973 Dec 1;138(6):1426–1442. doi: 10.1084/jem.138.6.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon A., Mitsuyasu R., Stevens R., Champlin R. E., Kimata H., Gale R. P. Designed transfer of specific immune responses with bone marrow transplantation. J Clin Invest. 1986 Oct;78(4):959–967. doi: 10.1172/JCI112686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S., Park A. B., Nordin A. A. Immunoglobulin classes of antibody-forming cells in mice. I. Localized hemolysis-in-agar plaque-forming cells belonging to five immunoglobulin classes. J Immunol. 1970 Feb;104(2):483–494. [PubMed] [Google Scholar]
- Slade M. S., Simmons R. L., Yunis E., Greenberg L. J. Immunodepression after major surgery in normal patients. Surgery. 1975 Sep;78(3):363–372. [PubMed] [Google Scholar]
- Thiele C. J., Morrow C. D., Stevens R. H. Multiple subsets of anti-tetanus toxoid antibody-producing cells in human peripheral blood differ by size, expression of membrane receptors, and mitogen reactivity. J Immunol. 1981 Mar;126(3):1146–1153. [PubMed] [Google Scholar]
- Wimperis J. Z., Brenner M. K., Prentice H. G., Reittie J. E., Karayiannis P., Griffiths P. D., Hoffbrand A. V. Transfer of a functioning humoral immune system in transplantation of T-lymphocyte-depleted bone marrow. Lancet. 1986 Feb 15;1(8477):339–343. doi: 10.1016/s0140-6736(86)92315-9. [DOI] [PubMed] [Google Scholar]


