Abstract
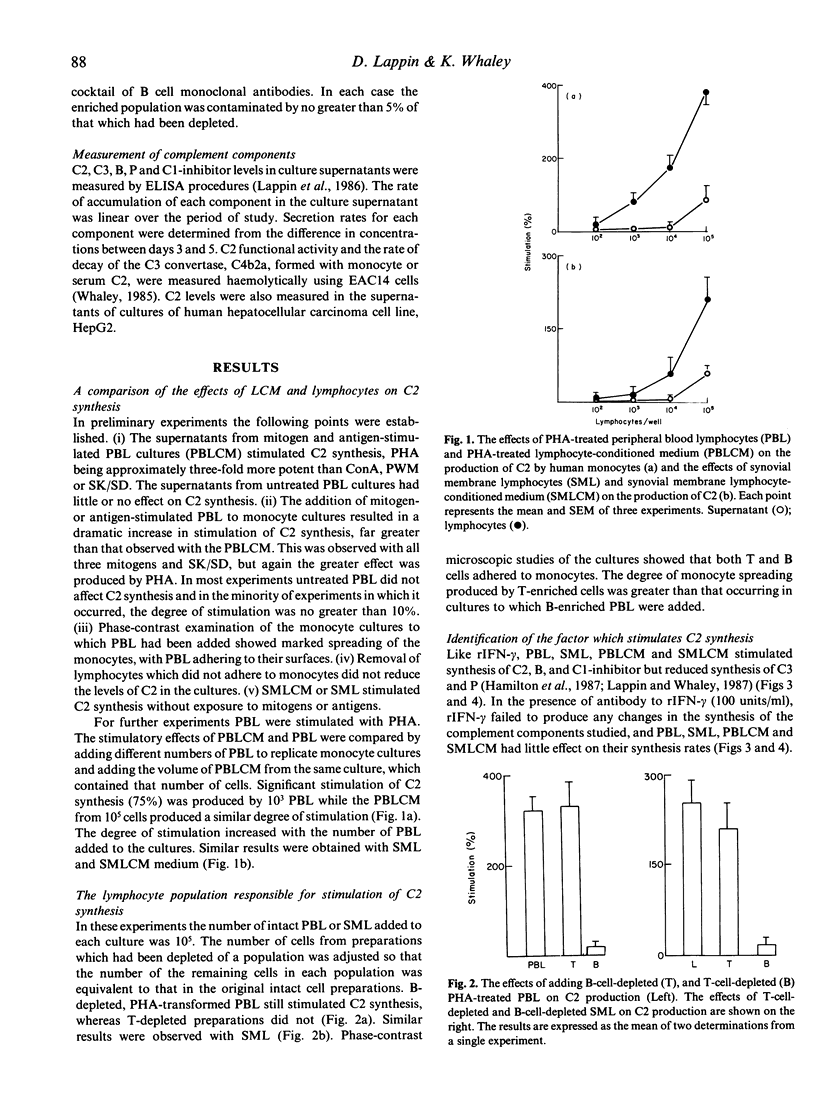
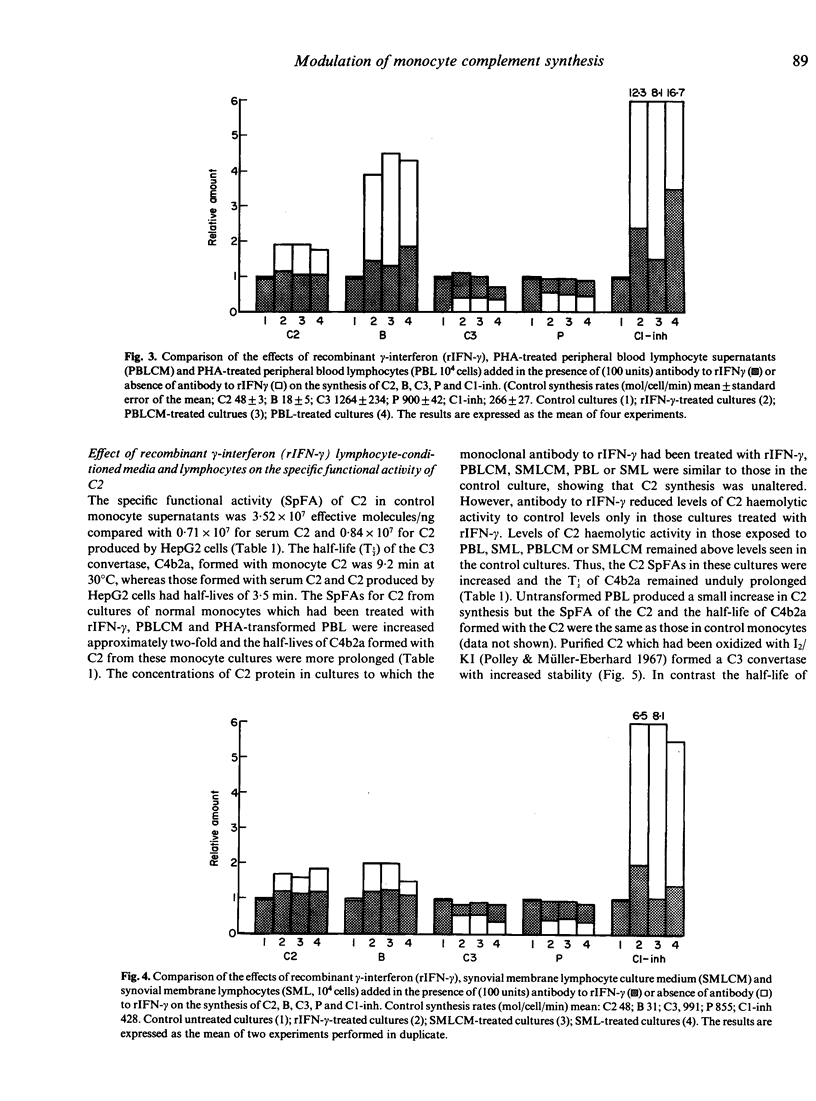
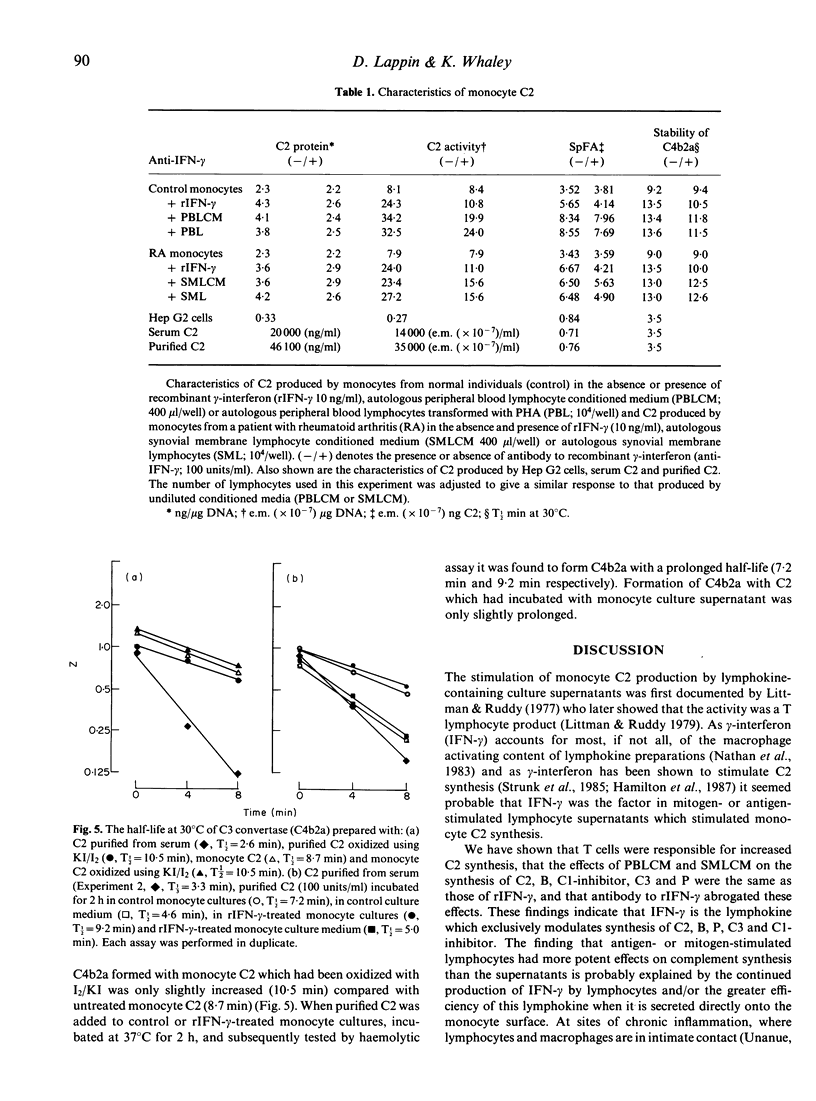
Culture supernatants from mitogen- and antigen-stimulated human peripheral blood lymphocytes (PBL), stimulated synthesis of the second complement component (C2) by human monocytes, but not as effectively as the stimulated PBL themselves, which adhered to the monocytes and caused marked spreading. In contrast to PBL, lymphocytes isolated from the synovial membranes (SML) of patients with rheumatoid arthritis and their culture supernatants were able to stimulate C2 synthesis without exposure to mitogens or antigens. Depletion of B and T populations showed that T cells were responsible for stimulation of C2 synthesis. Further studies of synthesis rates of C2, C3 factor B (B), C1 inhibitor, and properdin (P) were undertaken, and it was found that lymphocytes and their supernatants increased synthesis of C2, B and C1 inhibitor, and reduced synthesis of C3 and P. This profile of activity was identical to that produced by the addition of recombinant gamma-interferon (rIFN-gamma) to the cultures. Furthermore the addition of a monoclonal antibody to rIFN-gamma to cultures abrogated the effects of rIFN-gamma, and almost completely reversed the effects of lymphocytes and their supernatants. Thus it appears that gamma-interferon is the lymphocyte product which is responsible for the modulation of monocyte complement synthesis. The results of studies with synovial membrane lymphocytes raise the possibility that this process occurs in vivo. Monocyte C2 had a higher specific functional activity (SpFA) than serum C2 isolated from serum or C2 produced by HepG2 cells. Monocyte C2 formed a C3 convertase which had a longer half-life than that found with both serum C2 or HepG2 C2. Thus monocyte C2 behaves like oxidized C2. Monocytes exposed to rIFN-gamma, lymphocytes or lymphocyte-conditioned medium (LCM) produced C2 which had an even higher SpFA. Although antibody to IFN-gamma prevented any increase in C2 synthesis in monocyte cultures containing lymphocytes or LCM, C2 SpFA was still increased. Thus a second lymphocyte product is responsible for this 'oxidation' effect. This production of 'oxidized' C2 by monocytes and further 'oxidation' by the action of either lymphocytes or gamma-interferon might play a significant role in the perpetuation of complement activation at sites of inflammation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENDER M. A., PRESCOTT D. M. DNA synthesis and mitosis in cultures of human peripheral leukocytes. Exp Cell Res. 1962 Aug;27:221–229. doi: 10.1016/0014-4827(62)90225-2. [DOI] [PubMed] [Google Scholar]
- Cesarone C. F., Bolognesi C., Santi L. Improved microfluorometric DNA determination in biological material using 33258 Hoechst. Anal Biochem. 1979 Nov 15;100(1):188–197. doi: 10.1016/0003-2697(79)90131-3. [DOI] [PubMed] [Google Scholar]
- Einstein L. P., Schneeberger E. E., Colten H. R. Synthesis of the second component of complement by long-term primary cultures of human monocytes. J Exp Med. 1976 Jan 1;143(1):114–126. doi: 10.1084/jem.143.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton A. O., Jones L., Morrison L., Whaley K. Modulation of monocyte complement synthesis by interferons. Biochem J. 1987 Mar 15;242(3):809–815. doi: 10.1042/bj2420809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappin D., Hamilton A. D., Morrison L., Aref M., Whaley K. Synthesis of complement components (C3, C2, B and C1-inhibitor) and lysozyme by human monocytes and macrophages. J Clin Lab Immunol. 1986 Jul;20(3):101–105. [PubMed] [Google Scholar]
- Lappin D., Riches D. W., Damerau B., Whaley K. Cyclic nucleotides and their relationship to complement-component-C2 synthesis by human monocytes. Biochem J. 1984 Sep 1;222(2):477–486. doi: 10.1042/bj2220477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappin D., Whaley K. The role of ion channels and protein kinase C activation in the stimulation of complement protein synthesis. J Clin Lab Immunol. 1987 Oct;24(2):57–62. [PubMed] [Google Scholar]
- Littman B. H., Ruddy S. Monocyte complement stimulator: a T-lymphocyte product which stimulates synthesis of the second complement component (C2). Cell Immunol. 1979 Mar 15;43(2):388–397. doi: 10.1016/0008-8749(79)90183-7. [DOI] [PubMed] [Google Scholar]
- Littman B. H., Ruddy S. Production of the second component of complement by human monocytes: stimulation by antigen-activated lymphocytes or lymphokines. J Exp Med. 1977 May 1;145(5):1344–1352. doi: 10.1084/jem.145.5.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley M. J., Müller-Eberhard H. J. Enharncement of the hemolytic activity of the second component of human complement by oxidation. J Exp Med. 1967 Dec 1;126(6):1013–1025. doi: 10.1084/jem.126.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddy S., Austen K. F. A stoichiometric assay for the fourth component of complement in whole human serum using EAC'la-gp and functionally pure human second component. J Immunol. 1967 Dec;99(6):1162–1172. [PubMed] [Google Scholar]
- Strunk R. C., Cole F. S., Perlmutter D. H., Colten H. R. gamma-Interferon increases expression of class III complement genes C2 and factor B in human monocytes and in murine fibroblasts transfected with human C2 and factor B genes. J Biol Chem. 1985 Dec 5;260(28):15280–15285. [PubMed] [Google Scholar]
- Strunk R. C., Kunke K. S., Giclas P. C. Human peripheral blood monocyte-derived macrophages produce haemolytically active C3 in vitro. Immunology. 1983 May;49(1):169–174. [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Part Two: symbiotic relationship between lymphocytes and macrophages. Adv Immunol. 1981;31:1–136. doi: 10.1016/s0065-2776(08)60919-0. [DOI] [PubMed] [Google Scholar]
- Whaley K. Biosynthesis of the complement components and the regulatory proteins of the alternative complement pathway by human peripheral blood monocytes. J Exp Med. 1980 Mar 1;151(3):501–516. doi: 10.1084/jem.151.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung Laiwah A. C., Jones L., Hamilton A. O., Whaley K. Complement-subcomponent-C1-inhibitor synthesis by human monocytes. Biochem J. 1985 Feb 15;226(1):199–205. doi: 10.1042/bj2260199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ceulaer C., Papazoglou S., Whaley K. Increased biosynthesis of complement components by cultured monocytes, synovial fluid macrophages and skynovial membrane cells from patients with rheumatoid arthritis. Immunology. 1980 Sep;41(1):37–43. [PMC free article] [PubMed] [Google Scholar]


