Abstract
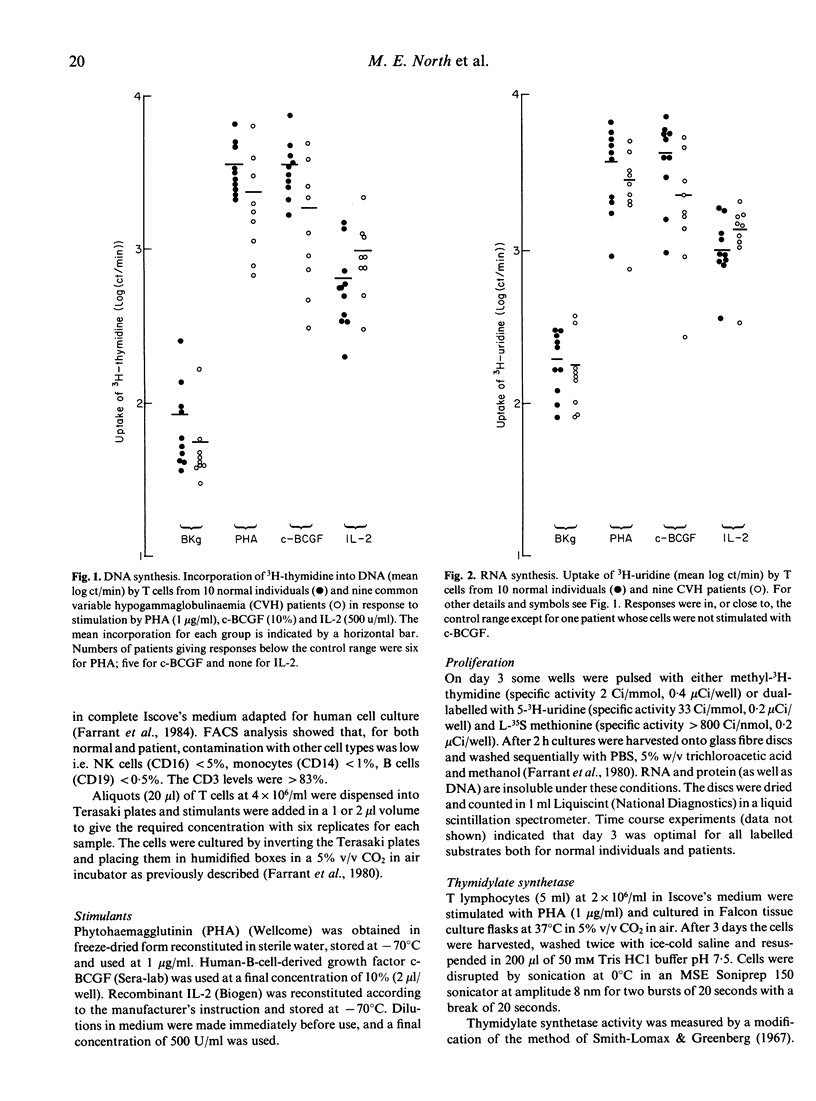
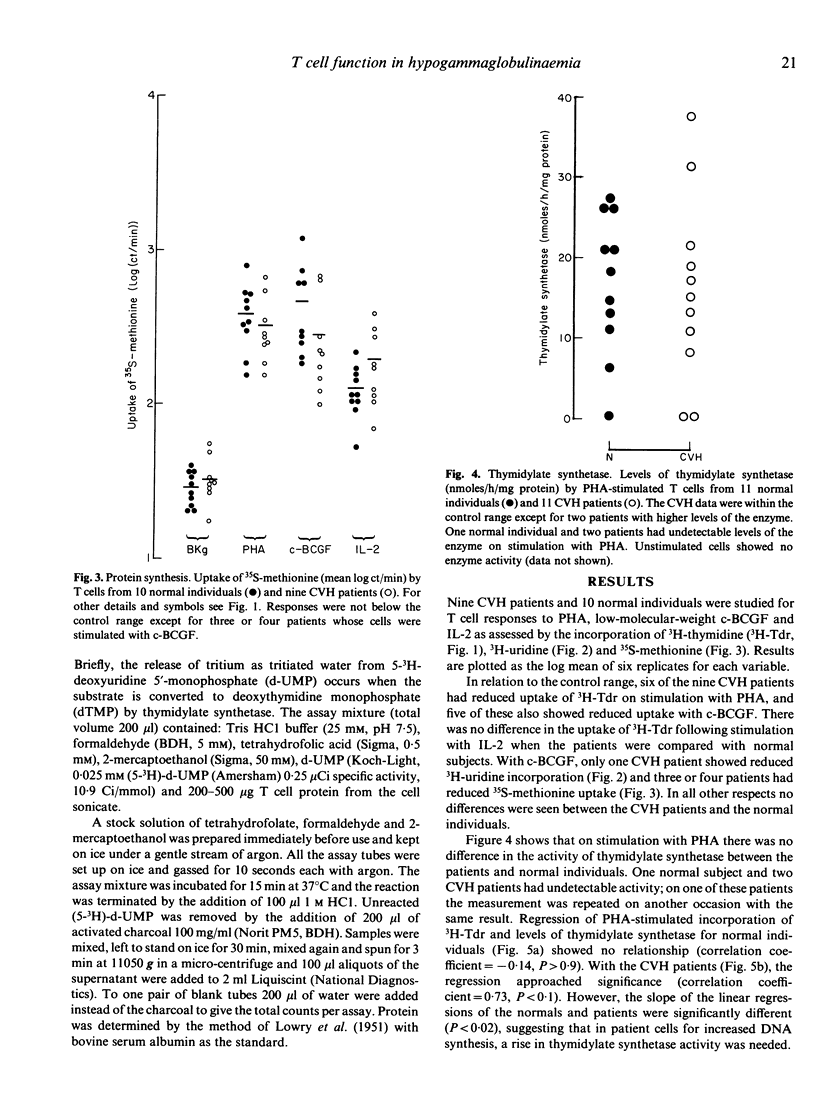
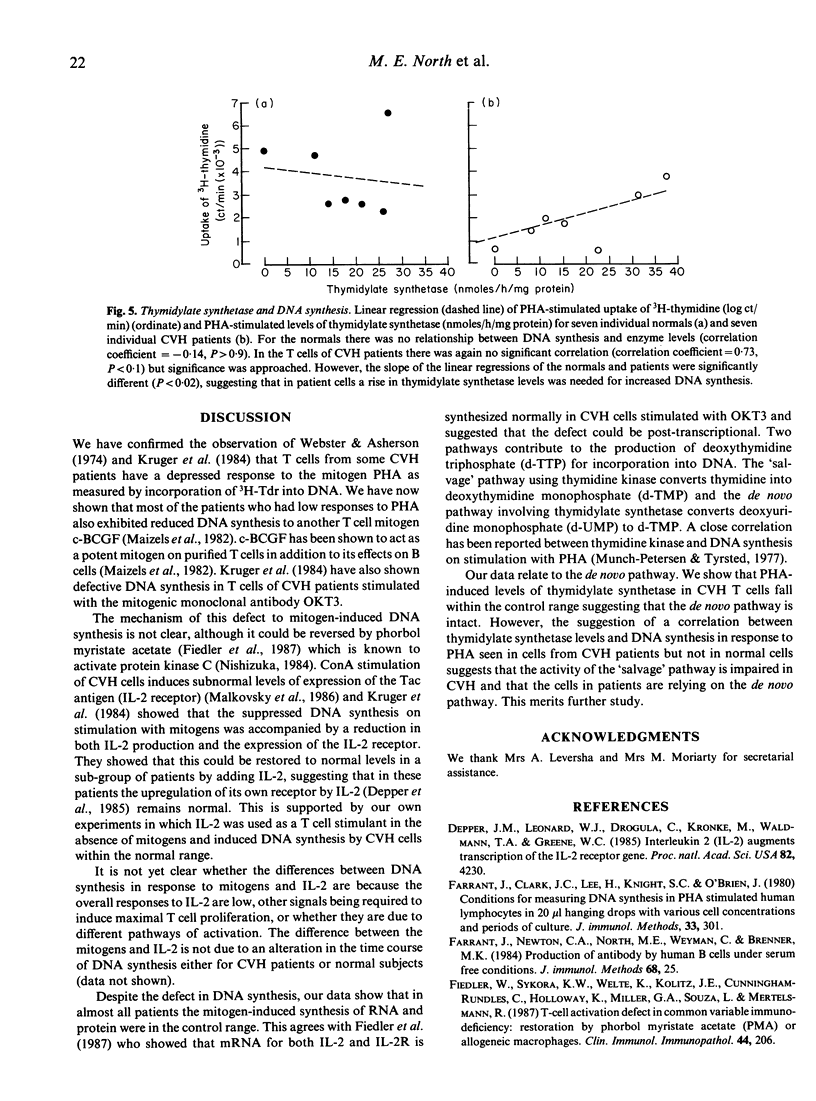
We have studied T cell defects in acquired 'common-variable' hypogammaglobulinaemia (CVH) by measuring the synthesis of DNA, RNA and protein in vitro in response to mitogens and to interleukin 2 (IL-2). We have confirmed that some patients have defective DNA synthesis in response to PHA and shown that this extends to responses to cell-derived B cell growth factor (c-BCGF) which is also mitogenic to T cells. DNA synthesis induced by IL-2 was not defective in these patients suggesting IL2-receptor induction is normal. The mitogen-related defect in DNA synthesis was not accompanied by any reduction in synthesis of RNA or of protein. Levels of the rate limiting enzyme (thymidylate synthetase EC 2.1.1.45) responsible for de novo DNA synthesis in the absence of endogenous thymidine were measured following PHA stimulation and found to be in the normal range. In the CVH patients (but not in normal individuals) the relationship between the levels of thymidylate synthetase and DNA synthesis in response to PHA approached significance, suggesting that this pathway becomes more important in CVH patients than in normal individuals perhaps because of defects in the thymidine 'salvage' pathway.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Depper J. M., Leonard W. J., Drogula C., Krönke M., Waldmann T. A., Greene W. C. Interleukin 2 (IL-2) augments transcription of the IL-2 receptor gene. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4230–4234. doi: 10.1073/pnas.82.12.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant J., Clark J. C., Lee H., Knight S. C., O'Brien J. Conditions for measuring DNA synthesis in PHA stimulated human lymphocytes in 20 microliters hanging drops with various cell concentrations and periods of culture. J Immunol Methods. 1980;33(4):301–312. doi: 10.1016/0022-1759(80)90001-0. [DOI] [PubMed] [Google Scholar]
- Farrant J., Newton C. A., North M. E., Weyman C., Brenner M. K. Production of antibody by human B cells under serum-free conditions. J Immunol Methods. 1984 Mar 30;68(1-2):25–34. doi: 10.1016/0022-1759(84)90133-9. [DOI] [PubMed] [Google Scholar]
- Fiedler W., Sykora K. W., Welte K., Kolitz J. E., Cunningham-Rundles C., Holloway K., Miller G. A., Souza L., Mertelsmann R. T-cell activation defect in common variable immunodeficiency: restoration by phorbol myristate acetate (PMA) or allogeneic macrophages. Clin Immunol Immunopathol. 1987 Aug;44(2):206–218. doi: 10.1016/0090-1229(87)90066-3. [DOI] [PubMed] [Google Scholar]
- Kruger G., Welte K., Ciobanu N., Cunningham-Rundles C., Ralph P., Venuta S., Feldman S., Koziner B., Wang C. Y., Moore M. A. Interleukin-2 correction of defective in vitro T-cell mitogenesis in patients with common varied immunodeficiency. J Clin Immunol. 1984 Jul;4(4):295–303. doi: 10.1007/BF00915297. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lomax M. I., Greenberg G. R. A new assay of thymidylate synthetase activity based on the release of tritium from deoxyuridylate-5-3-H. J Biol Chem. 1967 Jan 10;242(1):109–113. [PubMed] [Google Scholar]
- Maizel A., Sahasrabuddhe C., Mehta S., Morgan J., Lachman L., Ford R. Biochemical separation of a human B cell mitogenic factor. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5998–6002. doi: 10.1073/pnas.79.19.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkovský M., Jíra M., Gao L., Loveland B., Malkovska V., Dalgleish A. G., Webster A. D. Reduced expression of interleukin-2 receptors in hypogammaglobulinaemia: a possible cause of higher cancer incidence. Lancet. 1986 Jun 21;1(8495):1442–1443. doi: 10.1016/s0140-6736(86)91586-2. [DOI] [PubMed] [Google Scholar]
- Munch-Petersen B., Tyrsted G. Induction of thymidine kinases in phytohaemagglutinin-stimulated human lymphocytes. Biochim Biophys Acta. 1977 Oct 4;478(3):364–375. doi: 10.1016/0005-2787(77)90152-6. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Saiki O., Shimizu M., Saeki Y., Kishimoto S., Kishimoto T. Dissociation in the production of B cell-stimulating factors (BCGF and BCDF) and interleukin 2 by T cells from a common variable immunodeficient patient. J Immunol. 1984 Oct;133(4):1920–1924. [PubMed] [Google Scholar]
- Webster A. D., Asherson G. L. Identification and function of T cells in the peripheral blood of patients with hypogammaglobulinaemia. Clin Exp Immunol. 1974 Dec;18(4):499–504. [PMC free article] [PubMed] [Google Scholar]


