Abstract
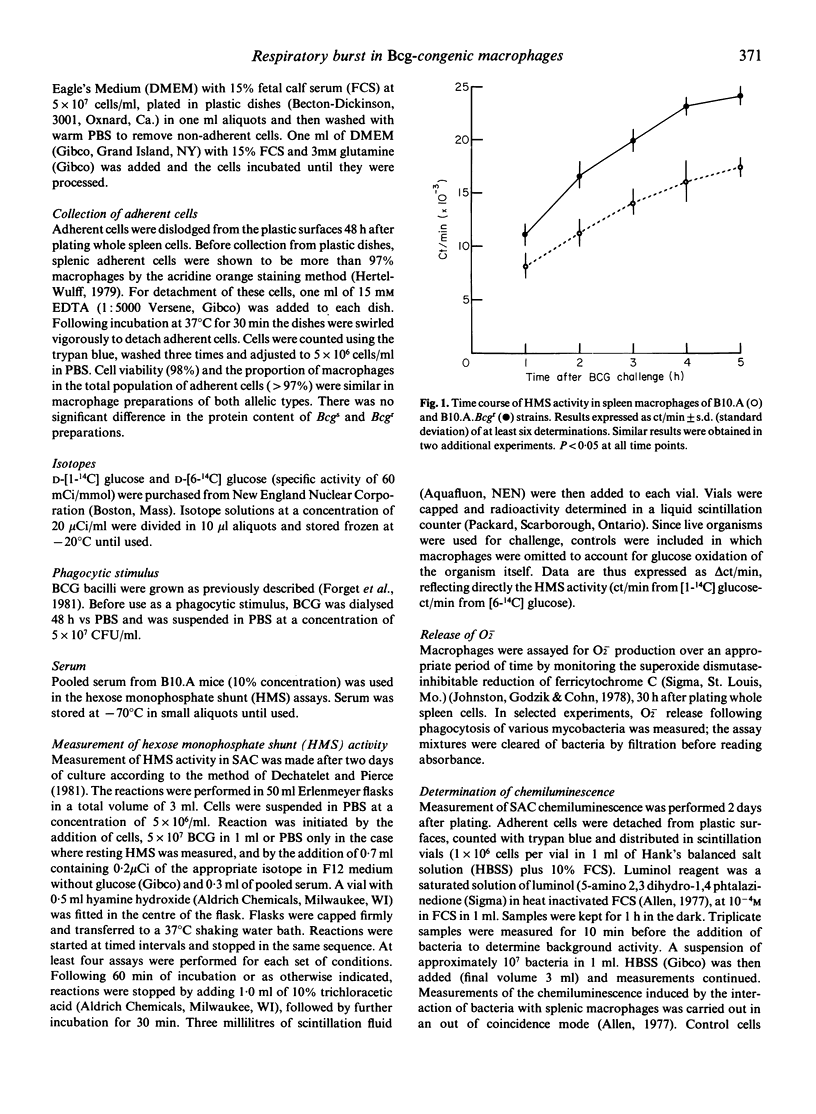
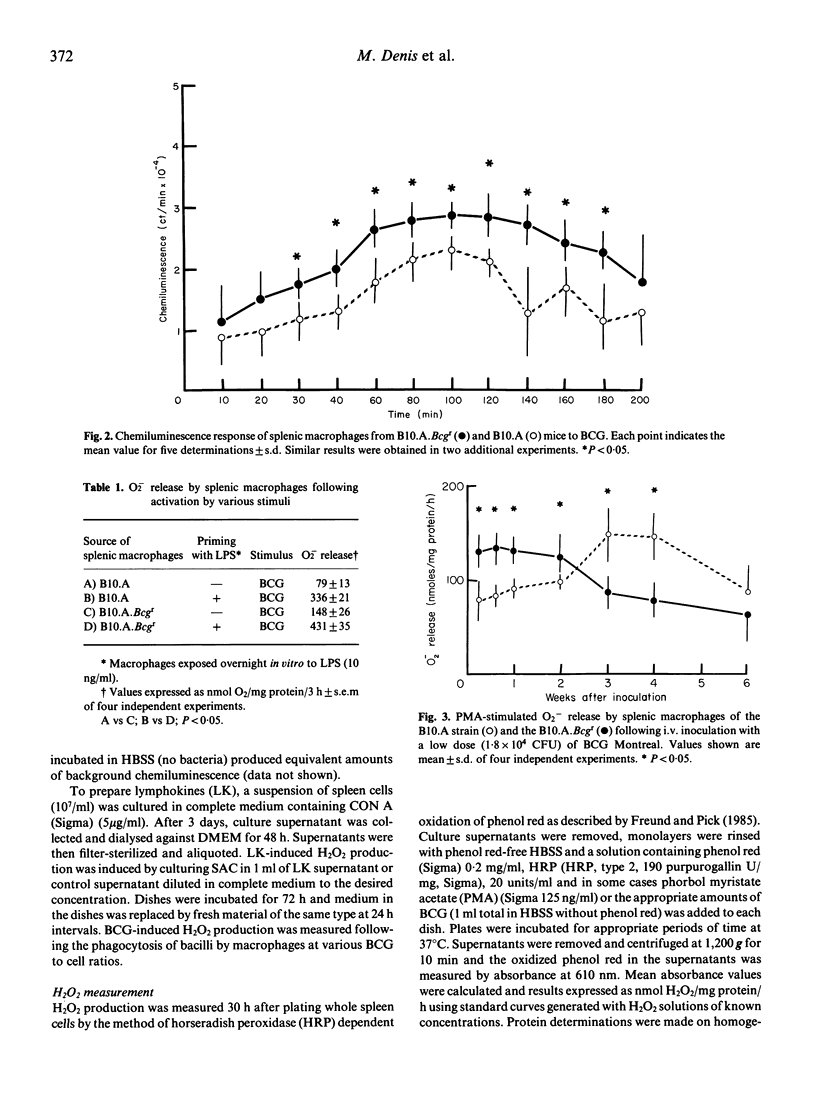
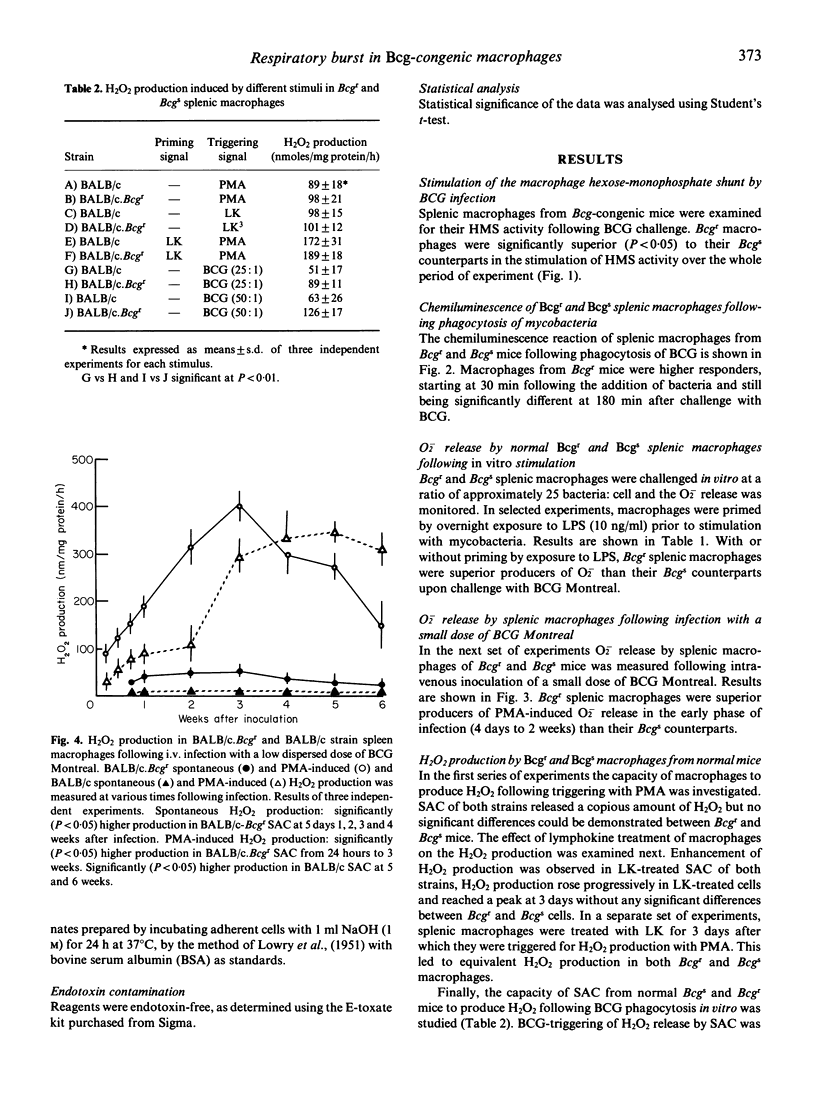
Macrophage respiratory burst, as assessed by H2O2 and O2- production, and HMS and chemiluminescence activity was investigated in a variety of conditions in macrophages from Bcg-congenic mice. Measurement of HMS and chemiluminescence in splenic macrophages challenged in vitro with BCG showed that the Bcgr cells were more stimulated by the challenge than their Bcgs counterparts. H2O2 production was measured in Bcgr and Bcgs splenic macrophages. PMA-triggering and LK-triggering were shown to stimulate a similar degree of H2O2 production Bcgr and Bcgs macrophages. In contrast, in vitro phagocytosis of BCG was shown to trigger superior production of H2O2 and O2- in the Bcgr splenic macrophages as compared to their Bcgs congenics. Finally, following the in vivo infection with BCG Montreal, Bcgr splenic macrophages were superior producers of H2O2 (both spontaneous and PMA-triggered) in the early phase of infection.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C. Evaluation of serum opsonic capacity by quantitating the initial chemiluminescent response from phagocytizing polymorphonuclear leukocytes. Infect Immun. 1977 Mar;15(3):828–833. doi: 10.1128/iai.15.3.828-833.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker P. R., Blackwell J. M., Bradley D. J. Expression of the natural resistance gene Lsh in resident liver macrophages. Infect Immun. 1984 Mar;43(3):1033–1040. doi: 10.1128/iai.43.3.1033-1040.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R. Initiation of the respiratory burst in human polymorphonuclear neutrophils: a critical review. J Reticuloendothel Soc. 1978 Jul;24(1):73–91. [PubMed] [Google Scholar]
- DeChatelet L. R., Lees C. J., Walsh C. E., Long G. D., Shirley P. S. Comparison of the calcium ionophore and phorbol myristate acetate on the initiation of the respiratory burst in human neutrophils. Infect Immun. 1982 Dec;38(3):969–974. doi: 10.1128/iai.38.3.969-974.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M., Forget A., Pelletier M., Skamene E. Pleiotropic effects of the Bcg gene. I. Antigen presentation in genetically susceptible and resistant congenic mouse strains. J Immunol. 1988 Apr 1;140(7):2395–2400. [PubMed] [Google Scholar]
- Denis M., Forget A., Pelletier M., Turcotte R., Skamene E. Control of the Bcg gene of early resistance in mice to infections with BCG substrains and atypical mycobacteria. Clin Exp Immunol. 1986 Mar;63(3):517–525. [PMC free article] [PubMed] [Google Scholar]
- Flesch I., Kaufmann S. H. Mycobacterial growth inhibition by interferon-gamma-activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis. J Immunol. 1987 Jun 15;138(12):4408–4413. [PubMed] [Google Scholar]
- Forget A., Skamene E., Gros P., Miailhe A. C., Turcotte R. Differences in response among inbred mouse strains to infection with small doses of Mycobacterium bovis BCG. Infect Immun. 1981 Apr;32(1):42–47. doi: 10.1128/iai.32.1.42-47.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund M., Pick E. The mechanism of action of lymphokines. VIII. Lymphokine-enhanced spontaneous hydrogen peroxide production by macrophages. Immunology. 1985 Jan;54(1):35–45. [PMC free article] [PubMed] [Google Scholar]
- Gangadharam P. R., Edwards C. K., 3rd Release of superoxide anion from resident and activated mouse peritoneal macrophages infected with Mycobacterium intracellulare. Am Rev Respir Dis. 1984 Nov;130(5):834–838. doi: 10.1164/arrd.1984.130.5.834. [DOI] [PubMed] [Google Scholar]
- Gros P., Skamene E., Forget A. Genetic control of natural resistance to Mycobacterium bovis (BCG) in mice. J Immunol. 1981 Dec;127(6):2417–2421. [PubMed] [Google Scholar]
- Jackett P. S., Aber V. R., Lowrie D. B. Virulence and resistance to superoxide, low pH and hydrogen peroxide among strains of Mycobacterium tuberculosis. J Gen Microbiol. 1978 Jan;104(1):37–45. doi: 10.1099/00221287-104-1-37. [DOI] [PubMed] [Google Scholar]
- Jackett P. S., Aber V. R., Lowrie D. B. Virulence of Mycobacterium tuberculosis and susceptibility to peroxidative killing systems. J Gen Microbiol. 1978 Aug;107(2):273–278. doi: 10.1099/00221287-107-2-273. [DOI] [PubMed] [Google Scholar]
- Jackett P. S., Andrew P. W., Aber V. R., Lowrie D. B. Hydrogen peroxide and superoxide release by alveolar macrophages from normal and BCG-vaccinated guinea-pigs after intravenous challenge with Mycobacterium tuberculosis. Br J Exp Pathol. 1981 Aug;62(4):419–428. [PMC free article] [PubMed] [Google Scholar]
- Johnson S. C., Zwilling B. S. Continuous expression of I-A antigen by peritoneal macrophages from mice resistant to Mycobacterium bovis (strain BCG). J Leukoc Biol. 1985 Nov;38(5):635–647. doi: 10.1002/jlb.38.5.635. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lepay D. A., Steinman R. M., Nathan C. F., Murray H. W., Cohn Z. A. Liver macrophages in murine listeriosis. Cell-mediated immunity is correlated with an influx of macrophages capable of generating reactive oxygen intermediates. J Exp Med. 1985 Jun 1;161(6):1503–1512. doi: 10.1084/jem.161.6.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissner C. R., Weinstein D. L., O'Brien A. D. Mouse chromosome 1 Ity locus regulates microbicidal activity of isolated peritoneal macrophages against a diverse group of intracellular and extracellular bacteria. J Immunol. 1985 Jul;135(1):544–547. [PubMed] [Google Scholar]
- Murray H. W. Cell-mediated immune response in experimental visceral leishmaniasis. II. Oxygen-dependent killing of intracellular Leishmania donovani amastigotes. J Immunol. 1982 Jul;129(1):351–357. [PubMed] [Google Scholar]
- Nathan C. F., Root R. K. Hydrogen peroxide release from mouse peritoneal macrophages: dependence on sequential activation and triggering. J Exp Med. 1977 Dec 1;146(6):1648–1662. doi: 10.1084/jem.146.6.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst M. J., Johnston R. B., Jr Increased production of superoxide anion by macrophages exposed in vitro to muramyl dipeptide or lipopolysaccharide. J Exp Med. 1980 Jan 1;151(1):101–114. doi: 10.1084/jem.151.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter M., O'Brien A. D., Skamene E., Gros P., Forget A., Kongshavn P. A., Wax J. S. A BALB/c congenic strain of mice that carries a genetic locus (Ityr) controlling resistance to intracellular parasites. Infect Immun. 1983 Jun;40(3):1234–1235. doi: 10.1128/iai.40.3.1234-1235.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stach J. L., Delgado G., Tchibozo V., Strobel M., Lagrange P. H. Natural resistance to mycobacteria: antimicrobial activity and reactive oxygen intermediate releasing functions of murine macrophages. Ann Immunol (Paris) 1984 Jul-Aug;135D(1):25–37. doi: 10.1016/s0769-2625(84)80152-x. [DOI] [PubMed] [Google Scholar]
- Walker L., Lowrie D. B. Killing of Mycobacterium microti by immunologically activated macrophages. Nature. 1981 Sep 3;293(5827):69–71. doi: 10.1038/293069a0. [DOI] [PubMed] [Google Scholar]


