Abstract
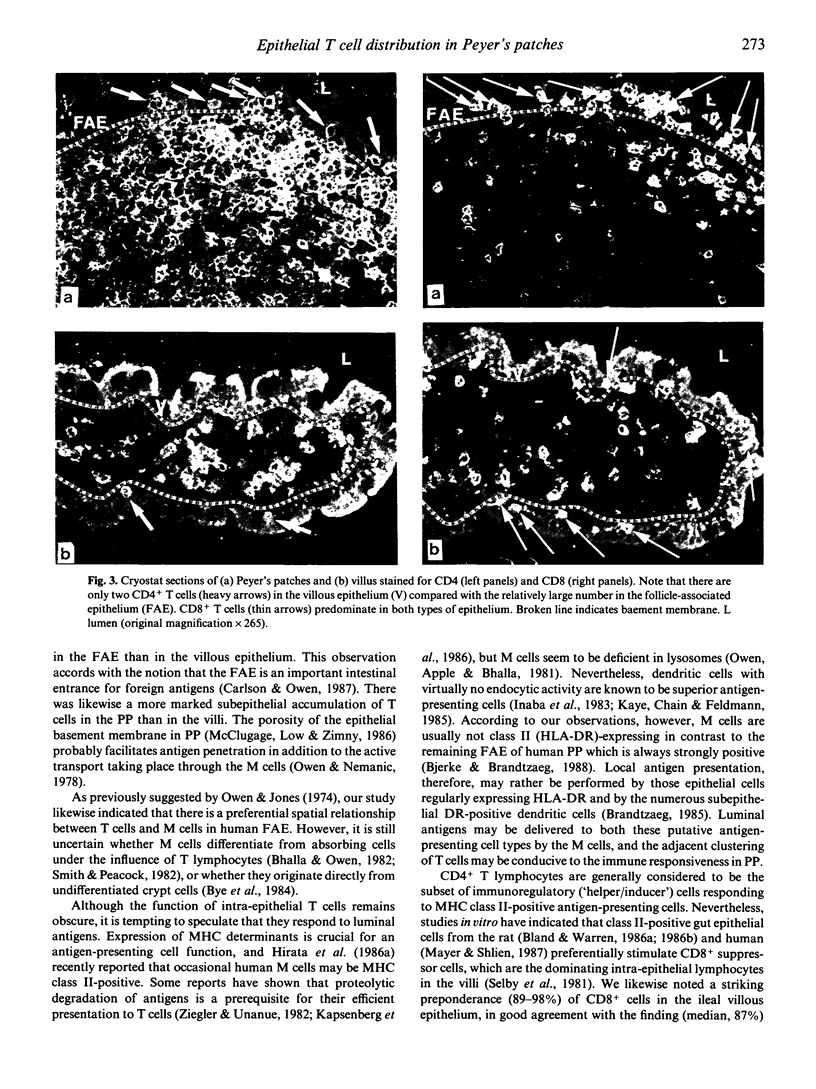
Immunohistochemical analyses performed on specimens of normal human ileum showed a significantly raised number of T cells in the follicle-associated epithelium (FAE) of Peyer's patches compared with the epithelium of distant villi. The T cells tended to be clustered in all layers of the FAE and were significantly more numerous adjacent to interruptions of the brush border (revealed by lack of staining for alkaline phosphatase). Such interruptions were taken to indicate 'membrane' (M) cells. Our findings therefore suggested a spatial relationship between M cells and the aggregation of T cells. The ratio of CD4+ to CD8+ T cells (approximately 4:10) was significantly higher in the FAE than in the villous epithelium (approximately 0.6:10). This suggested that the FAE may be involved to a greater extent in induction of 'helper' T cell functions, perhaps depending on luminal antigens transported by M cells, whereas the villous epithelium may be more involved in stimulation of 'suppressor' T cell functions as indicated by recent studies in vitro.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhalla D. K., Owen R. L. Cell renewal and migration in lymphoid follicles of Peyer's patches and cecum--an autoradiographic study in mice. Gastroenterology. 1982 Feb;82(2):232–242. [PubMed] [Google Scholar]
- Bjerke K., Brandtzaeg P. Lack of relation between expression of HLA-DR and secretory component (SC) in follicle-associated epithelium of human Peyer's patches. Clin Exp Immunol. 1988 Mar;71(3):502–507. [PMC free article] [PubMed] [Google Scholar]
- Bland P. W., Warren L. G. Antigen presentation by epithelial cells of the rat small intestine. I. Kinetics, antigen specificity and blocking by anti-Ia antisera. Immunology. 1986 May;58(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Bland P. W., Warren L. G. Antigen presentation by epithelial cells of the rat small intestine. II. Selective induction of suppressor T cells. Immunology. 1986 May;58(1):9–14. [PMC free article] [PubMed] [Google Scholar]
- Bockman D. E., Cooper M. D. Early lymphoepithelial relationships in human appendix. A combined light- and electron-microscopic study. Gastroenterology. 1975 May;68(5 Pt 1):1160–1168. [PubMed] [Google Scholar]
- Brandtzaeg P. Research in gastrointestinal immunology. State of the art. Scand J Gastroenterol Suppl. 1985;114:137–156. doi: 10.3109/00365528509093774. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Rognum T. O. Evaluation of tissue preparation methods and paired immunofluorescence staining for immunocytochemistry of lymphomas. Histochem J. 1983 Jul;15(7):655–689. doi: 10.1007/BF01002987. [DOI] [PubMed] [Google Scholar]
- Bye W. A., Allan C. H., Trier J. S. Structure, distribution, and origin of M cells in Peyer's patches of mouse ileum. Gastroenterology. 1984 May;86(5 Pt 1):789–801. [PubMed] [Google Scholar]
- Chu R. M., Glock R. D., Ross R. F. Gut-associated lymphoid tissues of young swine with emphasis on dome epithelium of aggregated lymph nodules (Peyer's patches) of the small intestine. Am J Vet Res. 1979 Dec;40(12):1720–1728. [PubMed] [Google Scholar]
- Faulk W. P., McCormick J. N., Goodman J. R., Yoffey J. M., Fudenberg H. H. Peyer's patches: morphologic studies. Cell Immunol. 1970 Nov;1(5):500–520. doi: 10.1016/0008-8749(70)90038-9. [DOI] [PubMed] [Google Scholar]
- Ferguson A., Parrott D. M. The effect of antigen deprivation on thymus-dependent and thymus-independent lymphocytes in the small intestine of the mouse. Clin Exp Immunol. 1972 Dec;12(4):477–488. [PMC free article] [PubMed] [Google Scholar]
- Hirata I., Austin L. L., Blackwell W. H., Weber J. R., Dobbins W. O., 3rd Immunoelectron microscopic localization of HLA-DR antigen in control small intestine and colon and in inflammatory bowel disease. Dig Dis Sci. 1986 Dec;31(12):1317–1330. doi: 10.1007/BF01299810. [DOI] [PubMed] [Google Scholar]
- Hirata I., Berrebi G., Austin L. L., Keren D. F., Dobbins W. O., 3rd Immunohistological characterization of intraepithelial and lamina propria lymphocytes in control ileum and colon and in inflammatory bowel disease. Dig Dis Sci. 1986 Jun;31(6):593–603. doi: 10.1007/BF01318690. [DOI] [PubMed] [Google Scholar]
- Huitfeldt H. S., Brandtzaeg P. Various keratin antibodies produce immunohistochemical staining of human myocardium and myometrium. Histochemistry. 1985;83(5):381–389. doi: 10.1007/BF00509196. [DOI] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M., Van Voorhis W. C., Muramatsu S. Dendritic cells are critical accessory cells for thymus-dependent antibody responses in mouse and in man. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6041–6045. doi: 10.1073/pnas.80.19.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapsenberg M. L., Teunissen M. B., Stiekema F. E., Keizer H. G. Antigen-presenting cell function of dendritic cells and macrophages in proliferative T cell responses to soluble and particulate antigens. Eur J Immunol. 1986 Apr;16(4):345–350. doi: 10.1002/eji.1830160405. [DOI] [PubMed] [Google Scholar]
- Kawanishi H., Ozato K., Strober W. The proliferative response of cloned Peyer's patch switch T cells to syngeneic and allogeneic stimuli. J Immunol. 1985 Jun;134(6):3586–3591. [PubMed] [Google Scholar]
- Kaye P. M., Chain B. M., Feldmann M. Nonphagocytic dendritic cells are effective accessory cells for anti-mycobacterial responses in vitro. J Immunol. 1985 Mar;134(3):1930–1934. [PubMed] [Google Scholar]
- Marsh M. N. Studies of intestinal lymphoid tissue. III. Quantitative analyses of epithelial lymphocytes in the small intestine of human control subjects and of patients with celiac sprue. Gastroenterology. 1980 Sep;79(3):481–492. [PubMed] [Google Scholar]
- Mayer L., Shlien R. Evidence for function of Ia molecules on gut epithelial cells in man. J Exp Med. 1987 Nov 1;166(5):1471–1483. doi: 10.1084/jem.166.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClugage S. G., Low F. N., Zimny M. L. Porosity of the basement membrane overlying Peyer's patches in rats and monkeys. Gastroenterology. 1986 Nov;91(5):1128–1133. doi: 10.1016/s0016-5085(86)80007-5. [DOI] [PubMed] [Google Scholar]
- Owen R. L., Bhalla D. K. Cytochemical analysis of alkaline phosphatase and esterase activities and of lectin-binding and anionic sites in rat and mouse Peyer's patch M cells. Am J Anat. 1983 Oct;168(2):199–212. doi: 10.1002/aja.1001680207. [DOI] [PubMed] [Google Scholar]
- Owen R. L., Jones A. L. Epithelial cell specialization within human Peyer's patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology. 1974 Feb;66(2):189–203. [PubMed] [Google Scholar]
- Owen R. L. Sequential uptake of horseradish peroxidase by lymphoid follicle epithelium of Peyer's patches in the normal unobstructed mouse intestine: an ultrastructural study. Gastroenterology. 1977 Mar;72(3):440–451. [PubMed] [Google Scholar]
- Rowiński J., Lamprecht J., Siciński P. Non-random distribution of intraepithelial lymphoid cells in follicle-associated epithelium of Peyer's patches in mice. J Anat. 1984 Aug;139(Pt 1):21–32. [PMC free article] [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Goldstein G., Jewell D. P. T lymphocyte subsets in human intestinal mucosa: the distribution and relationship to MHC-derived antigens. Clin Exp Immunol. 1981 Jun;44(3):453–458. [PMC free article] [PubMed] [Google Scholar]
- Smith M. W., Jarvis L. G., King I. S. Cell proliferation in follicle-associated epithelium of mouse Peyer's patch. Am J Anat. 1980 Oct;159(2):157–166. doi: 10.1002/aja.1001590204. [DOI] [PubMed] [Google Scholar]
- Wilders M. M., Sminia T., Janse E. M. Ontogeny of non-lymphoid and lymphoid cells in the rat gut with special reference to large mononuclear Ia-positive dendritic cells. Immunology. 1983 Oct;50(2):303–314. [PMC free article] [PubMed] [Google Scholar]
- Wolf J. L., Rubin D. H., Finberg R., Kauffman R. S., Sharpe A. H., Trier J. S., Fields B. N. Intestinal M cells: a pathway for entry of reovirus into the host. Science. 1981 Apr 24;212(4493):471–472. doi: 10.1126/science.6259737. [DOI] [PubMed] [Google Scholar]
- Ziegler H. K., Unanue E. R. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Rosen L., Podjaski B., Bettmann I., Otto H. F. Observations on the ultrastructure and function of the so-called "microfold" or "membraneous" cells (M cells) by means of peroxidase as a tracer. Virchows Arch A Pathol Anat Histol. 1981;390(3):289–312. doi: 10.1007/BF00496560. [DOI] [PubMed] [Google Scholar]


