Abstract
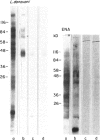
Sera from 29 patients with visceral leishmaniasis and 14 patients with cutaneous leishmaniasis were tested against a panel of nine nuclear antigens employing an enzyme-linked immunosorbent assay (ELISA). Anti- Sm, RNP, SS-A and SS-B antibodies were present in high titres in 83, 86, 36 and 73 per cent of the patients with visceral leishmaniasis and in 7, 14, 25 and 25 per cent of the patients with cutaneous leishmaniasis. One serum from a patient with visceral leishmaniasis which reacted strongly with Sm, RNP, SS-A and SS-B was examined by immunoblotting on extractable nuclear antigen from Hela cells. This serum binds to nine different antigenic bands (16, 23, 29, 30, 40, 50, 58, 100 and 115 kD). These same antigens were recognized by serum from a patient with systemic lupus erythematosus. The binding of visceral leishmaniasis serum antibodies to ribonucleoproteins was inhibited by prior incubation of serum with either leishmanial membrane antigens, from four different species of Leishmania, or intact cells of Leishmania donovani, implying molecular resemblance between common leishmanial antigens and ribonuclear antigens. It seems that appearance of autoantibodies to ribonucleoproteins in sera of patients infected with Leishmania is not only due to simply polyclonal activation of lymphocytes, but is also the result of a molecular mimicry between leishmanial antigens and ribonucleoproteins.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adu D., Williams D. G., Quakyi I. A., Voller A., Anim-Addo Y., Bruce-Tagoe A. A., Johnson G. D., Holborow E. J. Anti-ssDNA and antinuclear antibodies in human malaria. Clin Exp Immunol. 1982 Aug;49(2):310–316. [PMC free article] [PubMed] [Google Scholar]
- Bendixen G., Hadidi T., Manthorpe R., Permin H., Struckmann J., Wilk A., Zaghloul M. Antibodies against nuclear components in schistosomiasis. Results compared to values in patients with rheumatoid arthritis, systemic lupus erythematosus, and osteoarthrosis. Allergy. 1984 Feb;39(2):107–113. doi: 10.1111/j.1398-9995.1984.tb01941.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Böhme M. W., Evans D. A., Miles M. A., Holborow E. J. Occurrence of autoantibodies to intermediate filament proteins in human visceral leishmaniasis and their induction by experimental polyclonal B-cell activation. Immunology. 1986 Dec;59(4):583–588. [PMC free article] [PubMed] [Google Scholar]
- Carvalho E. M., Andrews B. S., Martinelli R., Dutra M., Rocha H. Circulating immune complexes and rheumatoid factor in schistosomiasis and visceral leishmaniasis. Am J Trop Med Hyg. 1983 Jan;32(1):61–68. doi: 10.4269/ajtmh.1983.32.61. [DOI] [PubMed] [Google Scholar]
- Carvalho E. M., Johnson W. D., Barreto E., Marsden P. D., Costa J. L., Reed S., Rocha H. Cell mediated immunity in American cutaneous and mucosal leishmaniasis. J Immunol. 1985 Dec;135(6):4144–4148. [PubMed] [Google Scholar]
- Castes M., Agnelli A., Verde O., Rondón A. J. Characterization of the cellular immune response in American cutaneous leishmaniasis. Clin Immunol Immunopathol. 1983 May;27(2):176–186. doi: 10.1016/0090-1229(83)90068-5. [DOI] [PubMed] [Google Scholar]
- Desjeux P., Santoro F., Afchain D., Loyens M., Capron A. Circulating immune complexes and anti-IgG antibodies in mucocutaneous leishmaniasis. Am J Trop Med Hyg. 1980 Mar;29(2):195–198. doi: 10.4269/ajtmh.1980.29.195. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishon T., Slutzky G. M., El-On J., Greenblatt C. L. Coagglutination and indirect hemagglutination in the detection of an excreted immunologically active substance from Leishmania. Isr J Med Sci. 1981 Apr;17(4):245–248. [PubMed] [Google Scholar]
- Galvão-Castro B., Sá Ferreira J. A., Marzochi K. F., Marzochi M. C., Coutinho S. G., Lambert P. H. Polyclonal B cell activation, circulating immune complexes and autoimmunity in human american visceral leishmaniasis. Clin Exp Immunol. 1984 Apr;56(1):58–66. [PMC free article] [PubMed] [Google Scholar]
- Houba V., Allison A. C. M-antiglobulins (rheumatoid-factor-like globulins) and other gamma-globulins in relation to tropical parasitic infections. Lancet. 1966 Apr 16;1(7442):848–852. doi: 10.1016/s0140-6736(66)90186-3. [DOI] [PubMed] [Google Scholar]
- Howard M. K., Miles M. A. Anti-tubulin antibodies in visceral leishmaniasis. Trans R Soc Trop Med Hyg. 1986;80(3):493–494. doi: 10.1016/0035-9203(86)90362-7. [DOI] [PubMed] [Google Scholar]
- Jaffe C. L., McMahon-Pratt D. Monoclonal antibodies specific for Leishmania tropica. I. Characterization of antigens associated with stage- and species-specific determinants. J Immunol. 1983 Oct;131(4):1987–1993. [PubMed] [Google Scholar]
- Jaffe C. L., McMahon-Pratt D. Serodiagnostic assay for visceral leishmaniasis employing monoclonal antibodies. Trans R Soc Trop Med Hyg. 1987;81(4):587–594. doi: 10.1016/0035-9203(87)90418-4. [DOI] [PubMed] [Google Scholar]
- Jaffe C. L., Zalis M. Use of purified parasite proteins from Leishmania donovani for the rapid serodiagnosis of visceral leishmaniasis. J Infect Dis. 1988 Jun;157(6):1212–1220. doi: 10.1093/infdis/157.6.1212. [DOI] [PubMed] [Google Scholar]
- Konikoff F., Shoenfeld Y., Isenberg D. A., Barrison I., Sobe T., Theodor E., Slor H. Anti-Rnp antibodies in chronic liver diseases. Clin Exp Rheumatol. 1987 Oct-Dec;5(4):359–361. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller R. A., Wener M. H., Harnisch J. P., Gilliland B. C. The limited spectrum of antinuclear antibodies in leprosy. J Rheumatol. 1987 Feb;14(1):108–110. [PubMed] [Google Scholar]
- Ott B. R., Libbey N. P., Ryter R. J., Trebbin W. M. Treatment of schistosome-induced glomerulonephritis. A case report and review of the literature. Arch Intern Med. 1983 Jul;143(7):1477–1479. doi: 10.1001/archinte.1983.00350070205035. [DOI] [PubMed] [Google Scholar]
- Pateraki E., Portocala R., Labrousse H., Guesdon J. L. Antiactin and antitubulin antibodies in canine visceral leishmaniasis. Infect Immun. 1983 Nov;42(2):496–500. doi: 10.1128/iai.42.2.496-500.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson P. Y. Microbial parasitism cross-reactive with host antigen: implications concerning loss of "self" tolerance and development of autoimmune disease. Recomb DNA Tech Bull. 1981 Sep;4(3):98–107. [PubMed] [Google Scholar]
- Pearson R. D., Wheeler D. A., Harrison L. H., Kay H. D. The immunobiology of leishmaniasis. Rev Infect Dis. 1983 Sep-Oct;5(5):907–927. doi: 10.1093/clinids/5.5.907. [DOI] [PubMed] [Google Scholar]
- Pearson R. D., de Alencar J. E., Romito R., Naidu T. G., Young A. C., Davis J. S., 4th Circulating immune complexes and rheumatoid factors in visceral leishmaniasis. J Infect Dis. 1983 Jun;147(6):1102–1102. doi: 10.1093/infdis/147.6.1102. [DOI] [PubMed] [Google Scholar]
- Rezai H. R., Ardehali S. M., Amirhakimi G., Kharazmi A. Immunological features of kala-azar. Am J Trop Med Hyg. 1978 Nov;27(6):1079–1083. doi: 10.4269/ajtmh.1978.27.1079. [DOI] [PubMed] [Google Scholar]
- Schnur L. F., Zuckerman A., Greenblatt C. L. Leishmanial serotypes as distinguished by the gel diffusion of factors excreted in vitro and in vivo. Isr J Med Sci. 1972 Jul;8(7):932–942. [PubMed] [Google Scholar]
- Schooley R. T., Haynes B. F., Payling-Wright C. R., Grouse J. E., Dolin R., Fauci A. S. Mechanism of Epstein-Barr virus-induced human B-lymphocyte activation. Cell Immunol. 1980 Dec;56(2):518–525. doi: 10.1016/0008-8749(80)90126-4. [DOI] [PubMed] [Google Scholar]
- Sela O., el-Roeiy A., Isenberg D. A., Kennedy R. C., Colaco C. B., Pinkhas J., Shoenfeld Y. A common anti-DNA idiotype in sera of patients with active pulmonary tuberculosis. Arthritis Rheum. 1987 Jan;30(1):50–56. doi: 10.1002/art.1780300107. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y., Hsu-Lin S. C., Gabriels J. E., Silberstein L. E., Furie B. C., Furie B., Stollar B. D., Schwartz R. S. Production of autoantibodies by human-human hybridomas. J Clin Invest. 1982 Jul;70(1):205–208. doi: 10.1172/JCI110595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoenfeld Y., Rauch J., Massicotte H., Datta S. K., André-Schwartz J., Stollar B. D., Schwartz R. S. Polyspecificity of monoclonal lupus autoantibodies produced by human-human hybridomas. N Engl J Med. 1983 Feb 24;308(8):414–420. doi: 10.1056/NEJM198302243080802. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y., Segol G., Segol O., Neary B., Klajman A., Stollar B. D., Isenberg D. A. Detection of antibodies to total histones and their subfractions in systemic lupus erythematosus patients and their asymptomatic relatives. Arthritis Rheum. 1987 Feb;30(2):169–175. doi: 10.1002/art.1780300207. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y., Vilner Y., Coates A. R., Rauch J., Lavie G., Shaul D., Pinkhas J. Monoclonal anti-tuberculosis antibodies react with DNA, and monoclonal anti-DNA autoantibodies react with Mycobacterium tuberculosis. Clin Exp Immunol. 1986 Nov;66(2):255–261. [PMC free article] [PubMed] [Google Scholar]
- Shoenfeld Y., el-Roeiy A., Ben-Yehuda O., Pick A. I. Detection of anti-histone activity in sera of patients with monoclonal gammopathies. Clin Immunol Immunopathol. 1987 Feb;42(2):250–258. doi: 10.1016/0090-1229(87)90012-2. [DOI] [PubMed] [Google Scholar]
- Thorns C. J., Morris J. A. Common epitopes between mycobacterial and certain host tissue antigens. Clin Exp Immunol. 1985 Aug;61(2):323–328. [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub J., Gottlieb M., Weinbaum F. I. Leishmania tropica: association of a B-cell mitogen with hypergammaglobulinemia in mice. Exp Parasitol. 1982 Feb;53(1):87–96. doi: 10.1016/0014-4894(82)90095-9. [DOI] [PubMed] [Google Scholar]
- Yamagata H., Harley J. B., Reichlin M. Molecular properties of the Ro/SSA antigen and enzyme-linked immunosorbent assay for quantitation of antibody. J Clin Invest. 1984 Aug;74(2):625–633. doi: 10.1172/JCI111460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman A. Current status of the immunology of blood and tissue Protozoa. I. Leishmania. Exp Parasitol. 1975 Dec;38(3):370–400. doi: 10.1016/0014-4894(75)90123-x. [DOI] [PubMed] [Google Scholar]