Abstract
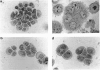
Two new cell lines with the phenotype of terminally differentiated B cells have been derived from the presentation bone marrow of a patient with plasma cell leukaemia. They express the same immunoglobulin (A1-kappa) as the original bone marrow cells. JJN-1 is an hypodiploid, slow-growing line with a plasmacytic morphology, which grows in medium with 15-20% fetal calf serum. When JJN-1 was stimulated with a supernatant ('ESG') containing B cell stimulatory factor 2 (BSF-2/IL-6), a hypotetraploid sub-line, JJN-2, was selectively stimulated. JJN-2 is dependent on ESG for survival. The stimulatory effect of ESG can be completely abrogated by an anti-BSF-2 monoclonal antibody. However, purified BSF-2 alone only produces sub-maximal stimulation of the lines. Both lines show complex karyotypic abnormalities, including 14q- and del(6q). JJN-1 and JJN-2 may be useful for the study of late B cell differentiation and for use as immunogens for the generation of anti-plasma cell monoclonal antibodies.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlogie B., Smith L., Alexanian R. Effective treatment of advanced multiple myeloma refractory to alkylating agents. N Engl J Med. 1984 May 24;310(21):1353–1356. doi: 10.1056/NEJM198405243102104. [DOI] [PubMed] [Google Scholar]
- Berger R., Bloomfield C. D., Sutherland G. R. Report of the Committee on Chromosome Rearrangements in Neoplasia and on Fragile Sites. Cytogenet Cell Genet. 1985;40(1-4):490–535. doi: 10.1159/000132181. [DOI] [PubMed] [Google Scholar]
- Callen D. F., Ford J. H. Chromosome abnormalities in chronic lymphocytic leukemia revealed by TPA as a mitogen. Cancer Genet Cytogenet. 1983 Sep;10(1):87–93. doi: 10.1016/0165-4608(83)90109-7. [DOI] [PubMed] [Google Scholar]
- Dewald G. W., Kyle R. A., Hicks G. A., Greipp P. R. The clinical significance of cytogenetic studies in 100 patients with multiple myeloma, plasma cell leukemia, or amyloidosis. Blood. 1985 Aug;66(2):380–390. [PubMed] [Google Scholar]
- Durie B. G., Vela E., Baum V., Leibovitz A., Payne C. M., Richter L. C., Grogan T. M., Trent J. M. Establishment of two new myeloma cell lines from bilateral pleural effusions: evidence for sequential in vivo clonal change. Blood. 1985 Sep;66(3):548–555. [PubMed] [Google Scholar]
- Gahrton G., Robèrt K. H., Friberg K., Zech L., Bird A. G. Nonrandom chromosomal aberrations in chronic lymphocytic leukemia revealed by polyclonal B-cell-mitogen stimulation. Blood. 1980 Oct;56(4):640–647. [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Garrett I. R., Durie B. G., Nedwin G. E., Gillespie A., Bringman T., Sabatini M., Bertolini D. R., Mundy G. R. Production of lymphotoxin, a bone-resorbing cytokine, by cultured human myeloma cells. N Engl J Med. 1987 Aug 27;317(9):526–532. doi: 10.1056/NEJM198708273170902. [DOI] [PubMed] [Google Scholar]
- Goldstein M., Hoxie J., Zembryki D., Matthews D., Levinson A. I. Phenotypic and functional analysis of B cell lines from patients with multiple myeloma. Blood. 1985 Aug;66(2):444–446. [PubMed] [Google Scholar]
- Hirano T., Yasukawa K., Harada H., Taga T., Watanabe Y., Matsuda T., Kashiwamura S., Nakajima K., Koyama K., Iwamatsu A. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986 Nov 6;324(6092):73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Jackson N., Ling N. R., Ball J., Bromidge E., Nathan P. D., Franklin I. M. An analysis of myeloma plasma cell phenotype using antibodies defined at the IIIrd International Workshop on Human Leucocyte Differentiation Antigens. Clin Exp Immunol. 1988 Jun;72(3):351–356. [PMC free article] [PubMed] [Google Scholar]
- Jobin M. E., Fahey J. L., Price Z. Long-term establishment of a human plasmacyte cell line derived from a patient with IgD multiple myeloma. I. Requirement of a plasmacyte-stimulating factor for the proliferation of myeloma cells in tissue culture. J Exp Med. 1974 Aug 1;140(2):494–507. doi: 10.1084/jem.140.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpas A., Fisher P., Swirsky D. Human plasmacytoma with an unusual karyotype growing in vitro and producing light-chain immunoglobulin. Lancet. 1982 Apr 24;1(8278):931–933. doi: 10.1016/s0140-6736(82)91933-x. [DOI] [PubMed] [Google Scholar]
- Kawano M., Hirano T., Matsuda T., Taga T., Horii Y., Iwato K., Asaoku H., Tang B., Tanabe O., Tanaka H. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988 Mar 3;332(6159):83–85. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- Lohmeyer J., Hadam M., Santoso S., Förster W., Schulz A., Pralle H. Establishment and characterization of a permanent human IgA2/kappa myeloma cell line. Br J Haematol. 1988 Jul;69(3):335–343. doi: 10.1111/j.1365-2141.1988.tb02371.x. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y., Moore G. E., Yagi Y., Pressman D. Production of free light chains of immunoglobulin by a hematopoietic cell line derived from a patient with multiple myeloma. Proc Soc Exp Biol Med. 1967 Aug-Sep;125(4):1246–1250. doi: 10.3181/00379727-125-32327. [DOI] [PubMed] [Google Scholar]
- Mingari M. C., Gerosa F., Carra G., Accolla R. S., Moretta A., Zubler R. H., Waldmann T. A., Moretta L. Human interleukin-2 promotes proliferation of activated B cells via surface receptors similar to those of activated T cells. Nature. 1984 Dec 13;312(5995):641–643. doi: 10.1038/312641a0. [DOI] [PubMed] [Google Scholar]
- Nilsson K., Bennich H., Johansson S. G., Pontén J. Established immunoglobulin producing myeloma (IgE) and lymphoblastoid (IgG) cell lines from an IgE myeloma patient. Clin Exp Immunol. 1970 Oct;7(4):477–489. [PMC free article] [PubMed] [Google Scholar]
- Schröder J., Vuopio P., Autio K. Chromosome changes in human chronic lymphocytic leukemia. Cancer Genet Cytogenet. 1981 Aug;4(1):11–21. doi: 10.1016/0165-4608(81)90003-0. [DOI] [PubMed] [Google Scholar]
- Seabright M. A rapid banding technique for human chromosomes. Lancet. 1971 Oct 30;2(7731):971–972. doi: 10.1016/s0140-6736(71)90287-x. [DOI] [PubMed] [Google Scholar]
- Taga T., Kawanishi Y., Hardy R. R., Hirano T., Kishimoto T. Receptors for B cell stimulatory factor 2. Quantitation, specificity, distribution, and regulation of their expression. J Exp Med. 1987 Oct 1;166(4):967–981. doi: 10.1084/jem.166.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme J., Opdenakker G., Simpson R. J., Rubira M. R., Cayphas S., Vink A., Billiau A., Van Snick J. Identification of the human 26-kD protein, interferon beta 2 (IFN-beta 2), as a B cell hybridoma/plasmacytoma growth factor induced by interleukin 1 and tumor necrosis factor. J Exp Med. 1987 Mar 1;165(3):914–919. doi: 10.1084/jem.165.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Berghe H., Parloir C., David G., Michaux J. L., Sokal G. A new characteristic karyotypic anomaly in lymphoproliferative disorders. Cancer. 1979 Jul;44(1):188–195. doi: 10.1002/1097-0142(197907)44:1<188::aid-cncr2820440131>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]