Abstract
PEANUT ALLERGY ACCOUNTS FOR THE MAJORITY of severe food-related allergic reactions. It tends to present early in life, and affected individuals generally do not outgrow it. In highly sensitized people, trace quantities can induce an allergic reaction. In this review, we will discuss the prevalence, clinical characteristics, diagnosis, natural history and management of peanut allergy.
Food allergies affect between 4% and 8% of children and between 1% and 2% of adults.1,2,3 They occur most often in young children and in individuals with a personal or family history of other atopic diseases.4,5,6 The majority of children outgrow their food allergies, and the foods can safely be reintroduced when they are older.7,8,9
The perceived prevalence of food allergies is substantially higher than the actual prevalence. Up to 30% of the general population believe they have a food allergy,10,11 and up to 30% of parents believe that their children have a food allergy.
Eight foods are responsible for more than 90% of food allergies: cow's milk, eggs, soy, wheat, peanuts, tree nuts (walnuts, hazelnuts, almonds, cashews, pecans and pistachios), fish and shellfish.12,13 All food allergies have the potential to induce anaphylaxis, but some foods are more likely than others to cause potentially life-threatening reactions. The food allergies most commonly associated with anaphylaxis (those to peanuts, tree nuts, fish and shellfish) are the ones least likely to resolve.
Data on the prevalence of food-induced anaphylaxis are limited because there is no requirement for mandatory reporting; however, of all emergency department visits because of anaphylactic events, food allergies account for the greatest proportion, and peanuts and tree nuts are responsible for the majority of serious events.14,15,16,17
Peanut allergy deserves particular attention. It accounts for the majority of severe food-related allergic reactions, it tends to present early in life, it does not usually resolve, and in highly sensitized people, trace quantities can induce an allergic reaction.5,18,19,20,21,22,23,24,25 In this review, we will discuss the prevalence, clinical characteristics, diagnosis, natural history and management of peanut allergy.
Prevalence
Several population-based studies have estimated the prevalence of peanut allergy.22,26,27,28 Tariq and colleagues22 followed a cohort on the Isle of Wight from birth until the age of 4 years. Families were asked about allergic reactions attributed to peanut or tree nut ingestion, skin prick tests were conducted, and peanut-specific IgE was measured. At age 4, 1.1% of the 1218 children were sensitized to peanuts, and 0.5% had had an allergic reaction to peanuts. An additional 1.2% of children were sensitized to tree nuts, with 0.2% having experienced an allergic reaction. On the basis of this and other studies that have reported similar prevalences,26,27,28 the estimated prevalence of peanut allergy in developed countries is between 0.6% and 1.0%. Recently, a follow-up study29 demonstrated that the prevalence of peanut allergy had increased to 1.5% on the Isle of Wight, which suggests that the problem is growing.
Pathophysiology
Although many foods can cause clinical syndromes in susceptible individuals, the allergic reaction provoked by peanuts is strictly an IgE-mediated type I hypersensitivity reaction. In such reactions, peanut-specific IgE antibodies bind to high-affinity receptors on mast cells and basophils. At least 7 peanut proteins have been identified that confer allergy. When peanut allergens penetrate mucosal barriers, cell-bound IgE and peanut allergens crosslink, which results in degranulation of preformed allergic mediators and subsequent cell activation. These cells may then produce a variety of cytokines and chemokines, which recruit other inflammatory cells and contribute to the IgE-mediated late-phase allergic response.30
Clinical characteristics
The clinical expression of peanut allergy is fairly predictable, and it has a tendency to be severe, although the severity may vary with different episodes of ingestion.5,18,20,21 The first allergic reaction to peanuts develops in most children between 14 and 24 months of age, and the first reaction most commonly occurs at home.20,21 According to a voluntary registry,20 about half of all children with peanut allergy have allergic manifestations in 1 target-organ system, 30% have symptoms in 2 systems, 10% to 15% in 3 systems, and 1% in 4 systems. The systems affected are listed in Table 1.
Table 1
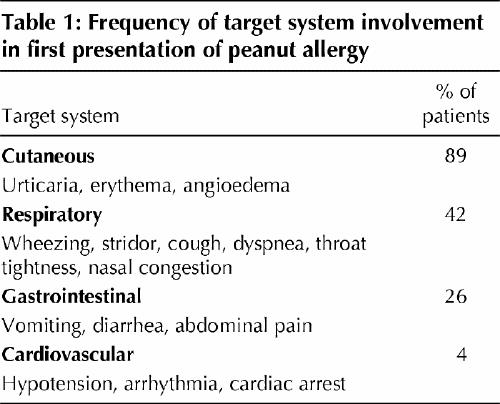
About 20% to 30% of food-induced anaphylactic events are characterized by a biphasic response, in which allergic symptoms recur 1 to 8 hours after the initial symptoms have resolved.31 Concomitant asthma and delay in administering epinephrine are risk factors for poor outcome of peanut anaphylaxis.18,20
One peanut contains about 200 mg of protein.32 In most people with peanut allergy, symptoms develop after substantially less than 1 peanut is ingested, and highly allergic people can react to trace amounts. In a study designed to determine the minimum dose of peanut protein capable of eliciting an allergic reaction in highly sensitized individuals, subjective symptoms were reported with doses as low as 100 μg, and objective signs were evident at 2 mg.25 Similar conclusions were drawn from a recent consensus publication that identified threshold doses of foods in people with allergies.33
In more than 70% of children with peanut allergy, symptoms develop at their first known exposure.5,20,21 Because IgE-mediated allergic reactions require an initial exposure to an allergen to induce immunologic sensitization, it seems apparent that occult exposure occurs. Possible routes of occult sensitization include fetal exposure to allergens ingested by the mother and infant exposure from breast milk.34,35,36 Although prospective intervention studies have resulted in conflicting reports about the role of breast milk in the expression of atopy, it seems prudent to recommend that mothers who are breast-feeding infants at risk for atopy avoid foods that could cause allergic reactions, particularly peanuts.35 An infant is deemed at risk for atopy if both parents, or one parent and a sibling, have atopic features.
Diagnosis
The diagnosis of a suspected food allergy begins with a medical history and a physical examination. It is confirmed with the detection of peanut-specific IgE, either by means of a skin prick test or fluoroenzyme immunoassay (Pharmacia ImmunoCAP-FEIA). When there is doubt about the diagnosis, oral food challenges can be performed. The characteristics of diagnostic tests for peanut allergy are listed in Table 2.
Table 2
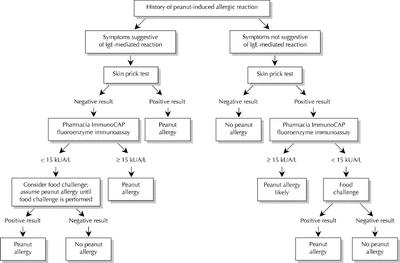
The medical history for a suspected IgE-mediated reaction should focus on the type and quantity of food ingested, the time of symptom onset, the severity and duration of symptoms, and the medical treatment administered. Personal or family details of atopy are also useful. Because most IgE-mediated reactions occur within 60 minutes after ingestion but may take as long as 4 hours after ingestion, symptoms occurring later than this are unlikely to have resulted from food allergy.21 Typically, minor allergic symptoms last less than 1 hour, but severe reactions may be protracted (Fig. 1).
Fig. 1: Algorithm for the diagnosis of peanut allergy.
IgE tests: in vivo and in vitro
Laboratory tests that identify specific IgE, such as the skin prick test and the ImmunoCAP-FEIA, should be conducted to confirm food allergy. A skin prick test with commercially prepared food extracts is a convenient and inexpensive method (a $20 vial of peanut extract is enough for 100 tests) of detecting IgE bound to dermal mast cells. A drop of glycerinated extract is placed on the forearm, and the skin is pricked through the drop. Positive (histamine) and negative (saline) controls are also tested. Results are available in 15 minutes; a positive result is one in which the wheal from the extract is at least 3 mm larger than that from the negative control.37,38 There is no age limit for food allergy skin testing, but very young and very old people are less likely to produce adequate control wheals.39 These tests are easily conducted and the results easily interpreted by most physicians. Ideally, all patients who require evaluation for food allergy should be seen by an allergist, particularly if they require food challenges. The skin prick test is safe to perform, but systemic reactions have been reported, especially when skin testing is performed at the time of active wheezing or when foods are tested intradermally.40,41,42,43,44,45 No fatal reactions have been reported. In general, skin tests have excellent sensitivity and negative predictive value but poor specificity and positive predictive value.46,47,48,49
The measurement of specific IgE antibodies in serum is useful for determining allergen-specific IgE in vitro. The ImmunoCAP-FEIA is a newer, preferred method and measures allergen-specific IgE bound to standardized allergens. Results are reported semiquantitatively, ranging from < 0.35 to > 100 kUA/L; values correlate with the probability of clinical reactivity but not with the severity of the reaction. Compared with the skin prick test, ImmunoCAP-FEIA is slightly less sensitive but may have a greater positive predictive value. Predictive values of 95% have been established for 4 foods: peanuts, eggs, milk and fish. For example, at a value of 15 kUA/L, 100% of subjects suspected of having peanut allergy had allergic symptoms after ingesting peanuts. According to published data, the positive and negative predictive values of ImmunoCAP-FEIA to peanuts at a level of 15 kUA/L or greater are 100% and 36%.49,50,51 A serum peanut-specific IgE exceeding this cutoff level would be considered positive, and an oral food challenge would not be warranted. Similar cutoff values have been established for a limited number of foods.
ImmunoCAP-FEIA can be performed when traditional skin prick testing is not possible, for example, when antihistamines have been taken or if severe skin conditions prohibit skin testing. Limitations of ImmunoCAP-FEIA include cost (about $10 to $15 per allergen), availability, lack of age-specific norms, limited data for the interpretation of results for foods other than those described above and a sensitivity of less than 100%. ImmunoCAP-FEIA is useful in determining which patients are best suited for food challenges and in diagnosing food allergy when the likelihood of reaction is high.49,50
Food challenges
Food challenges are the “gold standard” for diagnosing food allergies. These tests are performed when the medical history does not suggest an IgE-mediated reaction but the skin prick test result or ImmunoCAP-FEIA result is positive, when the history suggests an allergic reaction but confirmatory test results are negative, or when patients are evaluated for possible resolution of a known food allergy. Because the positive predictive value of a skin prick test is about 50%, food challenges are sometimes needed for final diagnosis. Increasingly, the ImmunoCAP-FEIA is being used to determine the best candidates for food challenges. Food challenges can be open, single blind or double blind. The most rigorous and least subjective method is the double-blind placebo-controlled food challenge, which is routinely used for research purposes.52,53,54,55,56
During a double-blind placebo-controlled food challenge, a person ingests incremental portions of food or placebo, hidden in a masking vehicle or gelatin capsule, at 15- to 30-minute intervals.57 Signs and symptoms of allergic reactions are documented before each dose. If unequivocal signs of an allergic reaction occur, the challenge is stopped and the necessary treatment administered. If no signs appear, the challenge continues until all portions of both the placebo and the suspect food are ingested. If all portions are ingested without an adverse reaction, the patient consumes a normal portion of the food to confirm tolerance. Because there is a risk of inducing a severe allergic reaction, the food challenge must be performed in a hospital by appropriately trained professionals, where access to emergency care and medications are readily available.58
Although the double-blind placebo-controlled food challenge is the “gold standard” to diagnose food allergy, it is time-consuming, requires close supervision by medical personnel and carries a risk of inducing a severe allergic reaction. Therefore, it is essential that patients are appropriately selected for the challenge, based on their clinical history and specific IgE test results.
Natural history
Until recently, peanut allergy was believed to persist indefinitely. In a longitudinal study of the natural history of peanut allergy, Bock23 contacted 32 of 46 eligible patients 2 to 14 years after their peanut allergy had been confirmed by a double-blind placebo-controlled food challenge: 75% had had an allergic reaction to accidentally ingested peanuts in the 5 years preceding contact (50% in the year preceding contact), and the remaining 25% had managed to completely avoid peanuts and had not experienced subsequent reactions. None of the people was known to be able to tolerate peanuts.
In an attempt to determine the severity of subsequent accidental peanut ingestion, Vander Leek and Bock59 conducted a prospective study on the natural history of peanut allergy. The parents of 83 children with peanut allergy were contacted annually and asked about accidental exposure to peanuts and the details of the ensuing reaction. Sixty children (72%) experienced allergic reactions during the study. The majority experienced potentially life-threatening reactions after accidental exposure, regardless of the nature of their initial reaction. However, 4 children did not have a reaction to accidental exposure, had low peanut-specific IgE levels and became tolerant to peanuts.59
From these studies, we can see that the majority of children with peanut allergy remain allergic indefinitely and are at high risk for accidental ingestion. The severity of their reactions can vary.
The possibility that peanut allergy can resolve has gained acceptance over the past several years. In 1998, Hourrihane and colleagues60 described the resolution of peanut allergy in 18% of people who had participated in oral peanut challenges. Using a case–control design, 15 children in whom peanut allergy had resolved were compared with 15 children matched for age and sex in whom peanut allergy persisted. There were no differences between the groups with respect to age at the time of initial reaction, severity of initial reaction or peanut-specific IgE levels. However, those whose allergies had resolved had smaller wheals on skin prick test at the time of reassessment and had fewer allergies to other foods. Although clinical and laboratory data were limited for many of the children, this study challenged the previously held belief that peanut allergy persists indefinitely in all patients.
Other groups have similarly concluded that peanut allergy can resolve in some people.24,61 Skolnick and coauthors24 reported on the frequency and characteristics of peanut allergy resolution in 223 people who were selected for an oral challenge because they had not had an allergic reaction to peanuts during the preceding year, they had low peanut-specific IgE levels or they met both criteria. Forty-eight (21%) of the children had no reaction from the challenge, which suggests that the peanut allergy had resolved. The children who outgrew their peanut allergy were more likely to have had a mild initial reaction, to have a significantly lower peanut-specific IgE level and to have a smaller wheal on skin prick test at the time of reassessment.
Management
Approaches to the treatment of an allergic reaction to peanuts are presented in Fig. 2. The mainstay of management is to educate people with peanut allergy and their families to avoid products containing peanuts, to recognize early signs of allergic reactions and to administer self-injectable epinephrine when indicated. Completely avoiding foods that contain peanuts is often difficult, as evidenced by the frequency with which accidental ingestion occurs, and the psychological burden weighs heavily on families.62,63
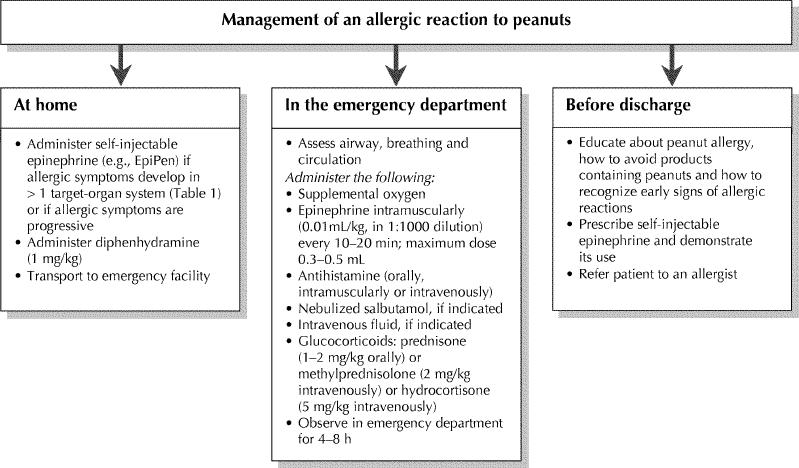
Fig. 2: Approaches to the management of peanut allergy.
People must be instructed to carefully read all ingredient labels when purchasing prepackaged foods and to ask about the risks of cross-contamination at restaurants and other public eateries. Children should be discouraged from sharing food at school or parties and should be encouraged to wash their hands after meals. School personnel should be made aware that a child has a food allergy and should be provided with photo identification of the child and a list of known allergies. Children with food allergies should always wear MedicAlert bracelets.
There is a considerable amount of resource material available to the public from Canadian information and advocacy groups, for example Anaphylaxis Canada (www.anaphylaxis.org) and the Allergy/Asthma Information Association (www.aaia.ca). Box 1 lists potential sources of peanut where exposure might not be readily anticipated.
Box 1.

The principal treatment of acute allergic reaction is epinephrine. Because delay in administering epinephrine is associated with poor outcome in anaphylactic reactions, and because the benefits of epinephrine far outweigh the risks in otherwise healthy people, prompt administration is recommended.18 People with peanut allergy should be prescribed self-injection devices such as EpiPen, EpiPen Jr and Ana-Kit, and they should be instructed to carry it with them at all times.18,20,21 In a survey of 101 families of children with food allergy, Sicherer and colleagues64 found that only 71% of the children had a self-injection epinephrine device available, 10% carried epinephrine devices beyond the expiration date, and only 32% were able to demonstrate its correct use. Patients, family members and caregivers should be instructed on the life-saving properties of self-injectable epinephrine.
Antihistamines can be used to treat food allergies, but they should serve as an adjunct to, not a replacement for, epinephrine. People with food allergies should be provided with an appropriate dose of a short-acting, first-generation antihistamine, such as diphenhydramine (1 mg/kg) or hydroxyzine (0.5 mg/kg).
After epinephrine is administered, all patients should be transported to a medical facility, where additional treatment can be administered if required. Because up to 30% of anaphylactic reactions have a biphasic component that can occur 1 to 8 hours after the onset of symptoms, patients should be observed for 4 to 8 hours after the onset of allergic symptoms in a facility that can provide emergency care.31
Future treatments
Therapeutic interventions are actively being pursued to prevent and manage food allergies. Humanized anti-IgE monoclonal antibody therapy and various modified immunotherapy regimes are under investigation, including cytokine modulation, plasmid-DNA immunotherapy, bioengineered proteins and peptide immunotherapy.
Humanized monoclonal IgE antibodies bind to natural IgE and prevent their binding to Fcε receptors on mast cells and basophils. When studied in people with allergic asthma, circulating IgE levels were reduced, and clinical improvement was reported.65,66,67,68 Studies involving individuals with food allergies are underway, and preliminary results are encouraging. In a recent study involving 84 adults with peanut allergy, promising data with the use of humanized monoclonal IgG1 antibody directed against IgE were reported; a significant increase in the threshold dose of peanut flour required to provoke allergic symptoms was demonstrated.69
Traditional immunotherapy has been attempted for peanut desensitization, but an unacceptably high rate of systemic reactions has limited its clinical application.70,71 The potential role for immunotherapy rests with modifications of the peanut protein, specific peptides or cytokine milieu. With the identification, sequencing and characterization of the main peanut proteins, new therapies are on the horizon.72,73,74 Until then, vigilance and the availability of self-injectable epinephrine remain the cornerstones of the management of peanut allergy.
Footnotes
This article has been peer reviewed.
Contributors: Dr. Al-Muhsen was responsible for the literature review and writing the initial draft of the manuscript. All authors contributed to the conception and design of the manuscript, and to revising and approving the final version of the manuscript.
Acknowledgement: Dr. Clarke is a Canadian Institutes of Health Research Investigator.
Competing interests: None declared.
Correspondence to: Dr. Rhoda S. Kagan, Rm. C-510, Montreal Children's Hospital, 2300 Tupper St., Montréal QC H3H 1P3; fax 514 412-4390; rhoda.kagan@muhc.mcgill.ca
References
- 1.Bock SA. Prospective appraisal of complaints of adverse reactions to foods in children during the first 3 years of life. Pediatrics 1987;79(5):683-8. [PubMed]
- 2.Jansen JJ, Kardinaal AF, Huijbers G, Vlieg-Boerstra BJ, Martens BP, Ockhuizen T. Prevalence of food allergy and intolerance in the adult Dutch population. J Allergy Clin Immunol 1994;93(2):446-56. [DOI] [PubMed]
- 3.Young E, Stoneham MD, Petruckevitch A, Barton J, Rona R. A population study of food intolerance. Lancet 1994;343(8906):1127-30. [DOI] [PubMed]
- 4.Hourihane JO, Dean TP, Warner JO. Peanut allergy in relation to heredity, maternal diet, and other atopic diseases: results of a questionnaire survey, skin prick testing, and food challenges. BMJ 1996;313:518-21. [DOI] [PMC free article] [PubMed]
- 5.Hourihane JO, Kilburn SA, Dean P, Warner JO. Clinical characteristics of peanut allergy. Clin Exp Allergy 1997;27(6):634-9. [PubMed]
- 6.Ewan PW. Clinical study of peanut and nut allergy in 62 consecutive patients: new features and associations. BMJ 1996;312:1074-8. [DOI] [PMC free article] [PubMed]
- 7.Host A. Clinical course of cow's milk protein allergy and intolerance. Pediatr Allergy Immunol 1998;9(Suppl 11):48-52. [PubMed]
- 8.Sampson HA, McCaskill CC. Food hypersensitivity and atopic dermatitis: evaluation of 113 patients. J Pediatr 1985;107(5):669-75. [DOI] [PubMed]
- 9.Host A, Halken S. A prospective study of cow milk allergy in Danish infants during the first 3 years of life. Clinical course in relation to clinical and immunological type of hypersensitivity reaction. Allergy 1990;45(8):587-96. [DOI] [PubMed]
- 10.Sloan AE, Powers ME. A perspective on popular perceptions of adverse reactions to foods. J Allergy Clin Immunol 1986;78(1 Pt 2):127-33. [DOI] [PubMed]
- 11.Woods RK, Stoney RM, Raven J, Walters EH, Abramson M, Thien FC. Reported adverse food reactions overestimate true food allergy in the community. Eur J Clin Nutr 2002;56(1):31-6. [DOI] [PubMed]
- 12.Sicherer SH, Sampson HA. Peanut and tree nut allergy. Curr Opin Pediatr 2000;12(6):567-73. [DOI] [PubMed]
- 13.Hefle SL, Nordlee JA, Taylor SL. Allergenic foods. Crit Rev Food Sci Nutr 1996;36(Suppl):S69-89. [DOI] [PubMed]
- 14.Kemp SF, Lockey RF, Wolf BL, Lieberman P. Anaphylaxis. A review of 266 cases. Arch Intern Med 1995;155(16):1749-54. [DOI] [PubMed]
- 15.Simons FER, Chad ZH, Gold M. Real-time reporting of anaphylaxis in infants, children, and adolescents by physician involved in the Canadian Pediatric Surveillance Program [abstract 536]. J Allergy Clin Immunol 2002;109(1 Pt 2):S181.
- 16.Mehra S, Salter J, Sussman G, Cairns J, Vadas P. A study of 32 food-related deaths from anaphylaxis: Ontario; 1986-2000 [abstract 537]. J Allergy Clin Immunol 2002;109(1 Pt 2):S181.
- 17.Macdougall CF, Cant AJ, Colver AF. How dangerous is food allergy in childhood? The incidence of severe and fatal allergic reactions across the UK and Ireland. Arch Dis Child 2002;86(4):236-9. [DOI] [PMC free article] [PubMed]
- 18.Sampson HA, Mendelson L, Rosen JP. Fatal and near-fatal anaphylactic reactions to food in children and adolescents. N Engl J Med 1992;327(6):380-4. [DOI] [PubMed]
- 19.Yocum M, Khan D. Assessment of patients who have experienced anaphylaxis: a 3-year survey. Mayo Clin Proc 1994;69(1):16-23. [DOI] [PubMed]
- 20.Sicherer SH, Furlong TJ, Munoz-Furlong A, Burks AW, Sampson HA. A voluntary registry for peanut and tree nut allergy: characteristics of the first 5149 registrants. J Allergy Clin Immunol 2001;108(1):128-32. [DOI] [PubMed]
- 21.Sicherer SH, Burks AW, Sampson HA. Clinical features of acute allergic reactions to peanut and tree nuts in children. Pediatrics 1998;102(1):e6. [DOI] [PubMed]
- 22.Tariq SM, Stevens M, Matthews S, Ridout S, Twiselton R, Hide DW. Cohort study of peanut and tree nut sensitisation by age of 4 years. BMJ 1996;313:514-7. [DOI] [PMC free article] [PubMed]
- 23.Bock SA, Atkins FM. The natural history of peanut allergy. J Allergy Clin Immunol 1989;83(5):900-4. [DOI] [PubMed]
- 24.Skolnick HS, Conover-Walker MK, Koerner CB, Sampson HA, Burks W, Wood RA. The natural history of peanut allergy. J Allergy Clin Immunol 2001; 107(2):367-74. [DOI] [PubMed]
- 25.Hourihane JB, Kilburn SA, Nordlee JA, Hefle SL, Taylor SL, Warner JO. An evaluation of the sensitivity of subjects with peanut allergy to very low doses of peanut protein: a randomized, double-blind, placebo-controlled food challenge study. J Allergy Clin Immunol 1997;100(5):596-600. [DOI] [PubMed]
- 26.Kanny G, Moneret-Vautrin DA, Flabbee J, Beaudouin E, Morisset M, Thevenin F. Population study of food allergy in France. J Allergy Clin Immunol 2001;108(1):133-40. [DOI] [PubMed]
- 27.Emmett SE, Angus FJ, Fry JS, Lee PN. Perceived prevalence of peanut allergy in Great Britain and its association with other atopic conditions and with peanut allergy in other household members. Allergy 1999;54(4):380-5. [DOI] [PubMed]
- 28.Sicherer SH, Munoz-Furlong A, Burks AW, Sampson HA. Prevalence of peanut and tree nut allergy in the US determined by a random digit dial telephone survey. J Allergy Clin Immunol 1999;103(4):559-62. [DOI] [PubMed]
- 29.Grundy J, Matthews S, Bateman B, Dean T, Arshad S. Rising prevalence of allergy to peanut in children: data from 2 sequential cohorts. J Allergy Clin Immunol 2002;110(5):784-9. [DOI] [PubMed]
- 30.Sampson HA. Food allergy. JAMA 1997;278(22):1888-94. [PubMed]
- 31.Stark BJ, Sullivan TJ. Biphasic and protracted anaphylaxis. J Allergy Clin Immunol 1986;78(1 Pt 1):76-83. [DOI] [PubMed]
- 32.Goldman M. Peanut allergy: How much peanut is too much? Baltimore (MD): Asthma and Allergy Foundation of America, Maryland–Greater Washington, DC, Chapter Newsletter; April–May 1998.
- 33.Taylor SL, Hefle SL, Bindslev-Jensen C, Bock SA, Burks AW Jr, Christie L, et al. Factors affecting the determination of threshold doses for allergenic foods: How much is too much? J Allergy Clin Immunol 2002;109(1):24-30. [DOI] [PubMed]
- 34.Frank L, Marian A, Visser M, Weinberg E, Potter PC. Exposure to peanuts in utero and in infancy and the development of sensitization to peanut allergens in young children. Pediatr Allergy Immunol 1999;10(1):27-32. [DOI] [PubMed]
- 35.Zeiger RS. Prevention of food allergy and atopic disease. J R Soc Med 1997; 90(Suppl 30):21-33. [DOI] [PMC free article] [PubMed]
- 36.Vadas P, Wai Y, Burks W, Perelman B. Detection of peanut allergens in breast milk of lactating women. JAMA 2001;285(13):1746-8. [DOI] [PubMed]
- 37.Bock SA, Lee WY, Remigio L, Holst A, May CD. Appraisal of skin tests with food extracts for diagnosis of food hypersensitivity. Clin Allergy 1978;8(6): 559-64. [DOI] [PubMed]
- 38.May CD. Objective clinical and laboratory studies of immediate hypersensitivity reactions to foods in asthmatic children. J Allergy Clin Immunol 1976;58 (4):500-15. [DOI] [PubMed]
- 39.Van Asperen PP, Kemp AS, Mellis CM. Skin test reactivity and clinical allergen sensitivity in infancy. J Allergy Clin Immunol 1984;73(3):381-6. [DOI] [PubMed]
- 40.Cantani A, Micera M. Can skin prick tests provoke severe allergic reactions? Eur Rev Med Pharmacol Sci 2000;4(5-6):145-8. [PubMed]
- 41.Lin MS, Tanner E, Lynn J, Friday GA Jr. Nonfatal systemic allergic reactions induced by skin testing and immunotherapy. Ann Allergy 1993;71(6):557-62. [PubMed]
- 42.Devenney I, Falth-Magnusson K. Skin prick tests may give generalized allergic reactions in infants. Ann Allergy Asthma Immunol 2000;85(6 Pt 1):457-60. [DOI] [PubMed]
- 43.Valyasevi MA, Maddox DE, Li JT. Systemic reactions to allergy skin tests. Ann Allergy Asthma Immunol 1999;83(2):132-6. [DOI] [PubMed]
- 44.Novembre E, Bernardini R, Bertini G, Massai G, Vierucci A. Skin-prick-test-induced anaphylaxis. Allergy 1995;50(6):511-3. [DOI] [PubMed]
- 45.Lockey R, Benedict L, Turkeltaub P, Bukantz S. Fatalities from immunotherapy (IT) and skin testing (ST). J Allergy Clin Immunol 1987;79:660-77. [DOI] [PubMed]
- 46.Rance F, Juchet A, Bremont F, Dutau G. Correlations between skin prick tests using commercial extracts and fresh foods, specific IgE, and food challenges. Allergy 1997;52(10):1031-5. [DOI] [PubMed]
- 47.Eigenmann PA, Sampson HA. Interpreting skin prick tests in the evaluation of food allergy in children. Pediatr Allergy Immunol 1998;9(4):186-91. [DOI] [PubMed]
- 48.Sampson HA, Albergo R. Comparison of results of skin tests, RAST, and double-blind placebo-controlled food challenges in children with atopic dermatitis. J Allergy Clin Immunol 1984;74:26-33. [DOI] [PubMed]
- 49.Sampson HA, Ho DG. Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. J Allergy Clin Immunol 1997;100(4):444-51. [DOI] [PubMed]
- 50.Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol 2001;107(5):891-6. [DOI] [PubMed]
- 51.Sampson HA. Clinical practice. Peanut allergy. N Engl J Med 2002;346(17): 1294-9. [DOI] [PubMed]
- 52.Bernstein M, Day JH, Welsh A. Double-blind food challenge in the diagnosis of food sensitivity in the adult. J Allergy Clin Immunol 1982;70(3):205-10. [DOI] [PubMed]
- 53.Bock SA, Atkins FM. Patterns of food hypersensitivity during sixteen years of double-blind, placebo-controlled food challenges. J Pediatr 1990;117(4):561-7. [DOI] [PubMed]
- 54.Sampson HA. Immunologically mediated food allergy: the importance of food challenge procedures. Ann Allergy 1988;60(3):262-9. [PubMed]
- 55.Burks AW, Mallory SB, Williams LW, Shirrell MA. Atopic dermatitis: clinical relevance of food hypersensitivity reactions. J Pediatr 1988;113(3):447-51. [DOI] [PubMed]
- 56.Kivity S, Dunner K, Marian Y. The pattern of food hypersensitivity in patients with onset after 10 years of age. Clin Exp Allergy 1994;24(1):19-22. [DOI] [PubMed]
- 57.Bock SA, Sampson HA, Atkins FM, Zeiger RS, Lehrer S, Sachs M, et al. Double-blind, placebo-controlled food challenge (DBPCFC) as an office procedure: a manual. J Allergy Clin Immunol 1988;82:986-97. [DOI] [PubMed]
- 58.Hamilos DL, Oppenheimer JJ, Nelson HS, Wenzel S, Driscoll S, Lockey RF, et al. Suggested approaches for research protocols involving the potential for life-threatening reactions. J Allergy Clin Immunol 1993;92:1101-20. [DOI] [PubMed]
- 59.Vander Leek TK, Liu AH, Stefanski K, Blacker B, Bock SA. The natural history of peanut allergy in young children and its association with serum peanut-specific IgE. J Pediatr 2000;137(6):749-55. [DOI] [PubMed]
- 60.Hourihane JO, Roberts SA, Warner JO. Resolution of peanut allergy: case–control study. BMJ 1998;316:1271-5. [DOI] [PMC free article] [PubMed]
- 61.Spergel JM, Beausoleil JL, Pawlowski NA. Resolution of childhood peanut allergy. Ann Allergy Asthma Immunol 2000;85(6 Pt 1):473-6. [DOI] [PubMed]
- 62.Sicherer SH, Noone SA, Munoz-Furlong A. The impact of childhood food allergy on quality of life. Ann Allergy Asthma Immunol 2001;87(6):461-4. [DOI] [PubMed]
- 63.Primeau MN, Kagan R, Joseph L, Lim H, Dufresne C, Duffy C, et al. The psychological burden of peanut allergy as perceived by adults with peanut allergy and the parents of peanut-allergic children. Clin Exp Allergy 2000;30(8):1135-43. [DOI] [PubMed]
- 64.Sicherer SH, Forman JA, Noone SA. Use assessment of self-administered epinephrine among food-allergic children and pediatricians. Pediatrics 2000; 105(2):359-62. [DOI] [PubMed]
- 65.Fahy JV, Fleming HE, Wong HH, Liu JT, Su JQ, Reimann J, et al. The effect of an anti-IgE monoclonal antibody on the early- and late-phase responses to allergen inhalation in asthmatic subjects. Am J Respir Crit Care Med 1997;155(6):1828-34. [DOI] [PubMed]
- 66.Casale TB, Bernstein IL, Busse WW, LaForce CF, Tinkelman DG, Stoltz RR, et al. Use of an anti-IgE humanized monoclonal antibody in ragweed-induced allergic rhinitis. J Allergy Clin Immunol 1997;100(1):110-21. [DOI] [PubMed]
- 67.MacGlashan DW Jr, Bochner BS, Adelman DC, Jardieu PM, Togias A, McKenzie-White J, et al. Down-regulation of Fc(epsilon)RI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol 1997;158(3):1438-45. [PubMed]
- 68.Milgrom H, Fick RB Jr, Su JQ, Reimann JD, Bush RK, Watrous ML, et al. Treatment of allergic asthma with monoclonal anti-IgE antibody. rhuMAb-E25 Study Group. N Engl J Med 1999;341(26):1966-73. [DOI] [PubMed]
- 69.Leung DY, Sampson HA, Yunginger JW, Burks AW Jr, Schneider LC, Wortel CH. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med 2003;348:986-93. [DOI] [PubMed]
- 70.Oppenheimer JJ, Nelson HS, Bock SA, Christensen F, Leung DY. Treatment of peanut allergy with rush immunotherapy. J Allergy Clin Immunol 1992; 90(2):256-62. [DOI] [PubMed]
- 71.Nelson HS, Lahr J, Rule R, Bock A, Leung D. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J Allergy Clin Immunol 1997;99(6 Pt 1):744-51. [DOI] [PubMed]
- 72.Shin DS, Compadre CM, Maleki SJ, Kopper RA, Sampson H, Huang SK, et al. Biochemical and structural analysis of the IgE binding sites on ara h1, an abundant and highly allergenic peanut protein. J Biol Chem 1998;273(22):13753-9. [DOI] [PubMed]
- 73.Stanley JS, King N, Burks AW, Huang SK, Sampson H, Cockrell G, et al. Identification and mutational analysis of the immunodominant IgE binding epitopes of the major peanut allergen Ara h 2. Arch Biochem Biophys 1997;342(2):244-53. [DOI] [PubMed]
- 74.Rabjohn P, Helm EM, Stanley JS, West CM, Sampson HA, Burks AW, et al. Molecular cloning and epitope analysis of the peanut allergen Ara h 3. J Clin Invest 1999;103(4):535-42. [DOI] [PMC free article] [PubMed]