Abstract
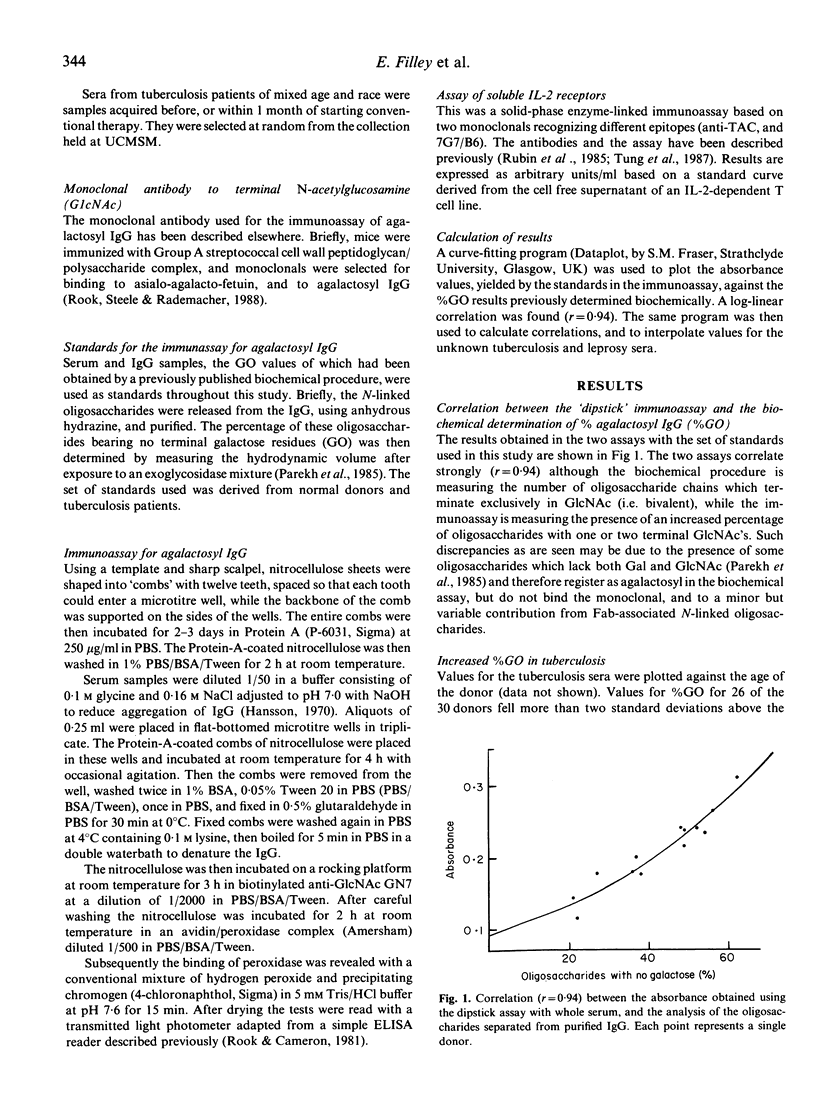
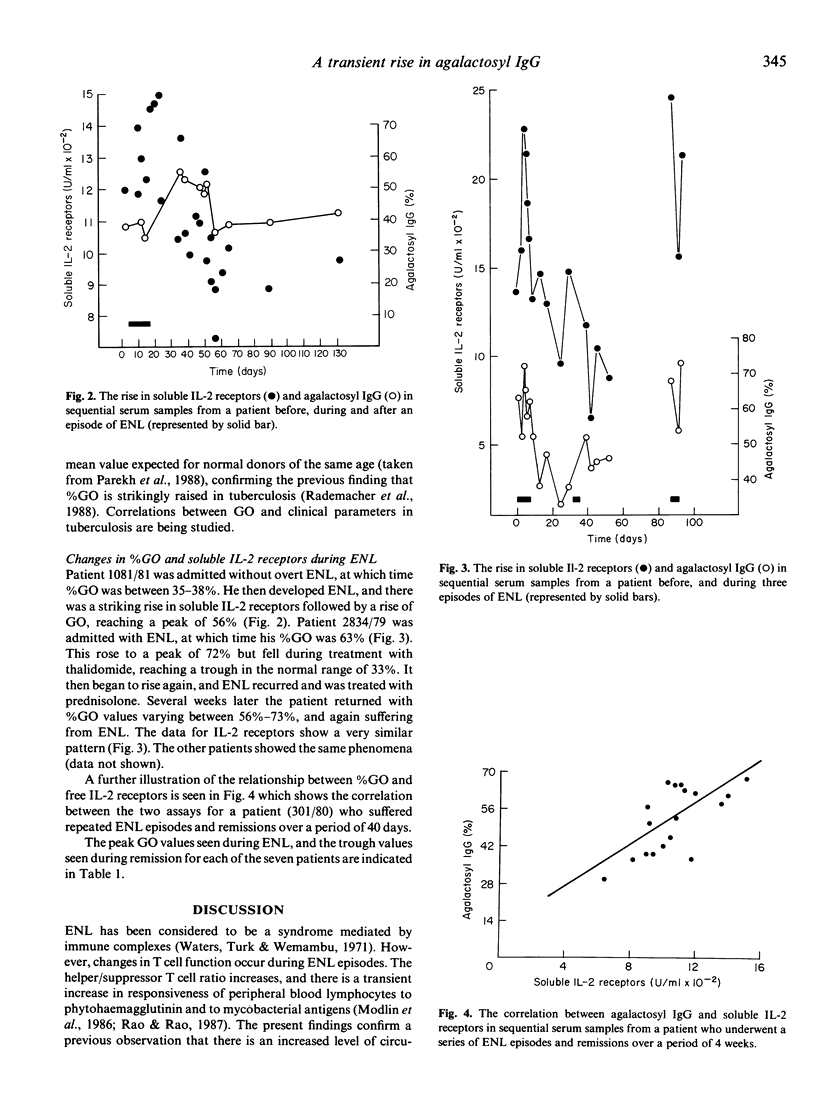
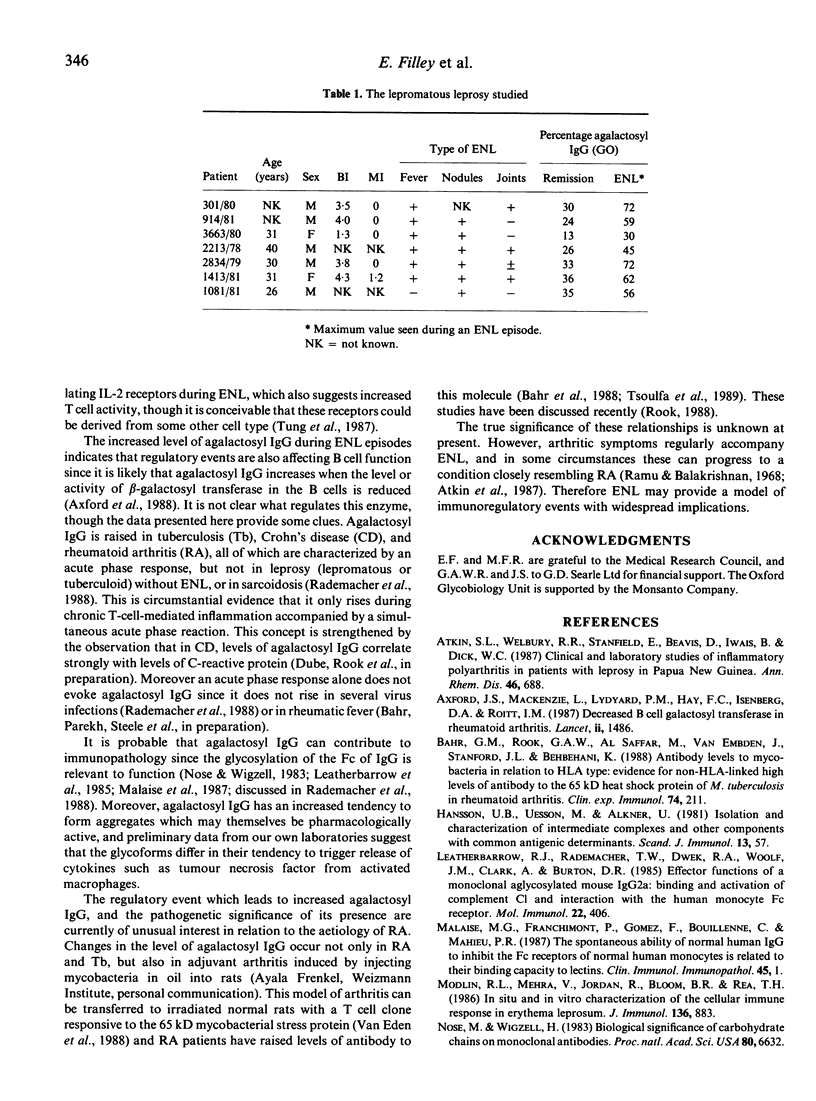
The proportion of oligosaccharide chains on the Fc fragment of IgG which terminate with N-acetylglucosamine and not galactose (%GO) has previously been shown to be raised in rheumatoid arthritis (RA), Crohn's disease (CD) and tuberculosis (Tb), but to be normal in sarcoidosis (SA), and in both lepromatous and tuberculoid leprosy. However we have now studied %GO in sequential serum samples collected from lepromatous leprosy patients undergoing episodes of erythema nodosum leprosum (ENL). During ENL %GO is transiently raised, and this rise parallels an increase in circulating interleukin 2 receptors (IL-2R). These findings confirm that changes in T cell function occur during ENL. Moreover it appears that %GO rises when there is, simultaneously, T-cell-mediated tissue damage and an acute phase response (RA, CD, Tb, ENL), but not when there is an acute phase response without major T cell involvement, or chronic T cell activity alone (SA, and tuberculoid leprosy). We suggest therefore that %GO is an indicator of a type of T cell activity with broad immunopathological implications.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkin S. L., Welbury R. R., Stanfield E., Beavis D., Iwais B., Dick W. C. Clinical and laboratory studies of inflammatory polyarthritis in patients with leprosy in Papua New Guinea. Ann Rheum Dis. 1987 Sep;46(9):688–690. doi: 10.1136/ard.46.9.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axford J. S., Mackenzie L., Lydyard P. M., Hay F. C., Isenberg D. A., Roitt I. M. Reduced B-cell galactosyltransferase activity in rheumatoid arthritis. Lancet. 1987 Dec 26;2(8574):1486–1488. doi: 10.1016/s0140-6736(87)92621-3. [DOI] [PubMed] [Google Scholar]
- Bahr G. M., Rook G. A., al-Saffar M., Van Embden J., Stanford J. L., Behbehani K. Antibody levels to mycobacteria in relation to HLA type: evidence for non-HLA-linked high levels of antibody to the 65 kD heat shock protein of M. bovis in rheumatoid arthritis. Clin Exp Immunol. 1988 Nov;74(2):211–215. [PMC free article] [PubMed] [Google Scholar]
- Hansson U. B., Uesson M., Alkner U. Isolation and characterization of intermediate complexes and other components with common antigenic determinants. Scand J Immunol. 1981;13(1):57–66. doi: 10.1111/j.1365-3083.1981.tb00111.x. [DOI] [PubMed] [Google Scholar]
- Malaise M. G., Franchimont P., Gomez F., Bouillenne C., Mahieu P. R. The spontaneous ability of normal human IgG to inhibit the Fc receptors of normal human monocytes is related to their binding capacity to lectins. Clin Immunol Immunopathol. 1987 Oct;45(1):1–16. doi: 10.1016/0090-1229(87)90106-1. [DOI] [PubMed] [Google Scholar]
- Modlin R. L., Mehra V., Jordan R., Bloom B. R., Rea T. H. In situ and in vitro characterization of the cellular immune response in erythema nodosum leprosum. J Immunol. 1986 Feb 1;136(3):883–886. [PubMed] [Google Scholar]
- Nose M., Wigzell H. Biological significance of carbohydrate chains on monoclonal antibodies. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6632–6636. doi: 10.1073/pnas.80.21.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh R. B., Dwek R. A., Sutton B. J., Fernandes D. L., Leung A., Stanworth D., Rademacher T. W., Mizuochi T., Taniguchi T., Matsuta K. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985 Aug 1;316(6027):452–457. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- Rademacher T. W., Parekh R. B., Dwek R. A., Isenberg D., Rook G., Axford J. S., Roitt I. The role of IgG glycoforms in the pathogenesis of rheumatoid arthritis. Springer Semin Immunopathol. 1988;10(2-3):231–249. doi: 10.1007/BF01857227. [DOI] [PubMed] [Google Scholar]
- Rao T. D., Rao P. R. Enhanced cell-mediated immune responses in erythema nodosum leprosum reactions of leprosy. Int J Lepr Other Mycobact Dis. 1987 Mar;55(1):36–41. [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Rook G. A., Cameron C. H. An inexpensive, portable, battery-operated photometer for the reading of ELISA tests in microtitration plates. J Immunol Methods. 1981;40(1):109–114. doi: 10.1016/0022-1759(81)90086-7. [DOI] [PubMed] [Google Scholar]
- Rook G. A. Rheumatoid arthritis, mycobacterial antigens and agalactosyl IgG. Scand J Immunol. 1988 Oct;28(4):487–493. doi: 10.1111/j.1365-3083.1988.tb01480.x. [DOI] [PubMed] [Google Scholar]
- Rook G. A., Steele J., Rademacher T. A monoclonal antibody raised by immunising mice with group A streptococci binds to agalactosyl IgG from rheumatoid arthritis. Ann Rheum Dis. 1988 Mar;47(3):247–250. doi: 10.1136/ard.47.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin L. A., Kurman C. C., Fritz M. E., Biddison W. E., Boutin B., Yarchoan R., Nelson D. L. Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. J Immunol. 1985 Nov;135(5):3172–3177. [PubMed] [Google Scholar]
- Tsoulfa G., Rook G. A., Van-Embden J. D., Young D. B., Mehlert A., Isenberg D. A., Hay F. C., Lydyard P. M. Raised serum IgG and IgA antibodies to mycobacterial antigens in rheumatoid arthritis. Ann Rheum Dis. 1989 Feb;48(2):118–123. doi: 10.1136/ard.48.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung K. S., Umland E., Matzner P., Nelson K., Schauf V., Rubin L., Wagner D., Scollard D., Vithayasai P., Vithayasai V. Soluble serum interleukin 2 receptor levels in leprosy patients. Clin Exp Immunol. 1987 Jul;69(1):10–15. [PMC free article] [PubMed] [Google Scholar]
- Waters M. F., Turk J. L., Wemambu S. N. Mechanisms of reactions in leprosy. Int J Lepr Other Mycobact Dis. 1971 Apr-Jun;39(2):417–428. [PubMed] [Google Scholar]
- van Eden W., Thole J. E., van der Zee R., Noordzij A., van Embden J. D., Hensen E. J., Cohen I. R. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature. 1988 Jan 14;331(6152):171–173. doi: 10.1038/331171a0. [DOI] [PubMed] [Google Scholar]


