The dramatic outbreak of severe acute respiratory syndrome (SARS) has led to the use of high-dose intravenous and oral ribavirin in patients affected with this disorder. Ribavirin, a nucleoside analogue with broad antiviral activity, was discovered in 1970 by ICN Pharmaceuticals. In Canada, ribavirin is licensed for the treatment of respiratory syncytial virus (RSV) infection in infants and, in combination with interferon α2b, hepatitis C. In view of the limited circumstances in which it is prescribed, most physicians are not familiar with its pharmacology, dosing and safety. In this article we summarize this information for the benefit of health care professionals who may be involved with patients receiving ribavirin for treatment or prevention of SARS. We also review emerging data on the potential efficacy of ribavirin against the SARS virus, a new mutant of coronavirus.
Clinical pharmacology
Ribavirin is a purine nucleoside analogue. Although its mechanism of action is still debated, it prevents replication of a large number of RNA and DNA viruses by inhibiting the enzyme inosine monophosphate dehydrogenase, which is required for the synthesis of guanosine triphosphate. The final step in this chain of events is lethal mutagenesis of the RNA genome.1 In vitro inhibition of RSV, influenza viruses and parainfluenza viruses is achieved at ribavirin concentrations of 3-10 μg/mL.
The plasma elimination of ribavirin occurs in 2 phases, the first with a relatively short half-life of 2 hours, the second with a much longer terminal half-life of 16–164 hours. The active metabolite of the drug, ribavirin triphosphate, concentrates in erythrocytes and leaches out slowly, with a half-life of 40 days. Ribavirin has 2 metabolic pathways: a reversible phosphorylation pathway and a degradative pathway involving deribosylation and amide hydrolysis.2 Ribavirin is eliminated primarily by renal excretion, and dose reductions are required in patients with renal insufficiency (Box 1).
Box 1.
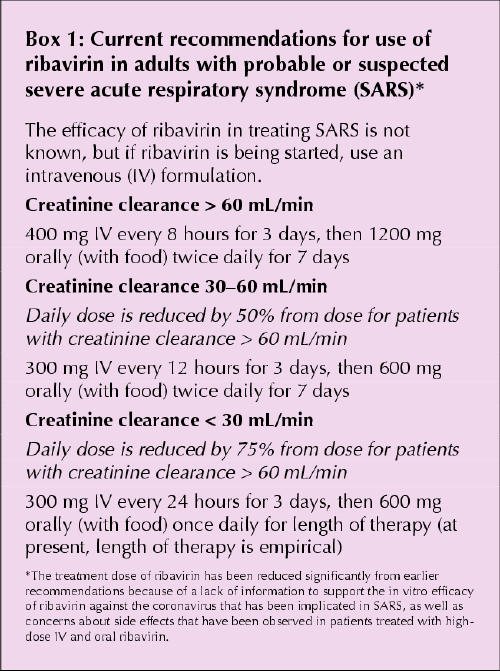
Ribavirin can be given orally (with an absolute bioavailability of 40% to 50%), intravenously or as an aerosol. In adults an oral dose of 600 mg yields peak plasma levels of 1.3 μg/mL, an intravenous dose of 1000 mg results in mean concentrations of 24 μg/mL, and the aerosol preparation appears in the plasma at levels of 0.2 to 1 μg/mL (in this case, levels of the drug in respiratory secretions can be up to 1000-fold higher). The recommended dosage regimens for adults with SARS are presented in Box 1, and recommendations for children with SARS appear in Box 2.
Box 2.

Applications
Aerosolized ribavirin was first used for RSV bronchiolitis and pneumonia in young hospitalized children, in whom it had a modest effect relative to that of placebo.3 It has been used mostly in young children with underlying risk factors (e.g., chronic lung disease or immunodeficiency) or severe lung disease (e.g., hypercapnea or hypoxemia) requiring ventilatory support.
The intravenous route has been used in treatment for Lassa fever, hemorrhagic fever with renal syndrome, hantavirus infection4 and severe adenovirus infection in immunocompromised children. The evidence for efficacy and safety with these uses is preliminary. The oral form of ribavirin, in conjunction with interferon α, is effective in patients with hepatitis C.5
Adverse effects
Administration of ribavarin as an aerosol is associated with nausea, headaches and, rarely, exacerbation or worsening of bronchospasm, both in infants and in care givers exposed to the drug. Ribavirin aerosol may cause rashes, conjunctivitis or opacities of contact lenses.
Systemic use of ribavirin (intravenous or oral administration) may cause dose-dependent anemia due to hemolysis and bone marrow suppression, both of which are reversible. Hemolytic anemia usually occurs after 10 days of therapy but may appear as early as 3 to 5 days after initiation of the drug; it is usually observed with doses of 1–2 g or higher.6 Patients with preexisting cardiac disease in whom anemia develops are at increased risk of deterioration of cardiac status. In HIV patients receiving other nucleoside analogues (as part of highly active antiretroviral therapy), in addition to ribavirin, elevated concentrations of lactate and pyruvate have been reported; it has been postulated that these findings were secondary to mitochondrial toxic effects associated with both drugs.7 In our own recent experience with SARS, we have seen hypocalcemia and hypomagnesemia associated with the use of high-dose ribavirin, and a recent report described an association between hypocalcemia even in patients who had been on lower doses of ribavirin as part of combination therapy for viral hepatitis.8 In addition to these electrolyte disturbances (hypocalcemia and hypomagnesemia), hyperammonemia and pancreatitis have also been reported.9,10 Central nervous system (CNS) effects, including CNS depression and mood changes, have been described; however, these effects are usually seen only in patients receiving concurrent interferon treatment for hepatitis C,11 which has been clearly associated with neuropsychiatric effects.
Box 3 lists patient groups in whom ribavarin should be avoided or modified, and Box 4 outlines appropriate monitoring for patients receiving ribavirin.
Box 3.

Box 4.

Drug interactions
Case reports suggest that the effects of warfarin may be inhibited during treatment with ribavirin, and this inhibition may continue for up to 1 month after discontinuation of the ribavirin therapy.12 Concurrent use of ribavirin and nucleoside analogue reverse-transcriptase inhibitors (e.g., zidovudine and stavudine) should be avoided because of the risk of lactic acidosis and other mitochondrial toxic effects, which may be higher in HIV patients who also have hepatitis C.
Teratogenic potential
Because it is a nucleoside analogue, ribavirin interferes with DNA and RNA replication and hence may affect the embryo. Rodent studies have shown teratogenicity with relatively low doses (1–10 mg/kg) but not in nonhuman primates at doses of 60–120 mg/kg.13 Pharmacokinetic modelling has suggested that aerosolized ribavirin may pose a teratogenic risk to health care workers.14
Although the true risk for teratogenic effects in humans is unknown, an industry-based registry at Schering Plough Inc. did not record a higher-than-expected teratogenic rate among several hundred pregnant patients with hepatitis C who were treated orally during their pregnancies.15 There are no data on ribavirin exposure through breast-feeding. Clearly, the current experience with intravenous ribavirin in the treatment of SARS is likely to result in much higher fetal exposure, and current recommendations include avoidance of the drug during pregnancy and lactation. Given the prolonged intracellular half-life and the current lack of knowledge in this regard, effective contraception should be practised by women who receive ribavirin, during treatment and for at least 6 months after treatment is completed. There are some concerns, as yet unproven, about potential effects on sperm; however, Schering's registry of almost 1000 men showed no malformations after exposure to the drug. Because ribavirin has been shown to be teratogenic only during embryogenesis (i.e., first trimester in humans), use of the drug in later stages of pregnancy may not increase the teratogenic risk.
If a woman needs ribavirin in pregnancy or has conceived within 6 months of her partner or herself being on the drug, she can be referred to the Motherisk Program at the Hospital for Sick Children (416 813-6780 or momrisk@sickkids.org) for counselling and follow-up.
Efficacy of ribavirin in SARS
Recent published reports from Hong Kong and Canada have shed light on early experience with ribavirin in combination with other antimicrobials, with or without steroids, in the treatment of SARS.
In Toronto, 7 patients were treated with oral oseltamivir, broad-spectrum antibiotics and intravenous ribavirin at 2 g loading dose, followed by 1 g every 6 hours for 4 days, then 500 mg every 8 hours for another 4 to 6 days.16 This dosing regimen was based on a recently published dose schedule for hemorrhagic fever viruses. Of these 7 patients, 1 died, 1 showed improvement on mechanical ventilation, and the other 5 improved within 5 days. Of note, in addition to receiving antivirals and antimicrobials, the 5 patients who improved had received earlier and more aggressive supportive care than the other 2 patients. These 5 have since recovered either completely (3 patients) or with mild dyspnea (2 patients) with 3 week follow-up. In contrast, of 3 patients who were treated only with broad-spectrum antibiotics, 2 died and 1 was still receiving ventilation in the intensive care unit at the time of writing (unpublished data). Since the publication of this case series16 at the end of March, anecdotal reports have been received about a smaller number of patients who did not receive ribavirin and who have recovered without incident.
In Hong Kong, 10 patients were treated empirically with ribavirin: 9 intravenously at 8 mg/kg every 8 hours and 1 orally at 1.2 g every 8 hours.17 Each patient received 1 of 2 corticosteroids, hydrocortisone or methylprednisolone. Treatment started 3 to 22 days (median 12.5 days) after the onset of symptoms. The authors reported resolution of fever and improvement in heart rate within 2 days of commencing treatment in 8 of the patients; the other 2 patients died of respiratory failure.
More data has further characterized the major outbreak of SARS in Hong Kong.18 In 138 patients with suspected SARS, a combination of ribavirin and corticosteroids was given to patients if fever persisted for more than 48 hours and blood counts showed leukopenia, thrombocytopenia or both. As in the other Hong Kong study,17 outcomes were not broken down according to which patients received ribavirin.
There are numerous methodological issues in these early reports that preclude any conclusions about the efficacy of ribavirin in the treatment of SARS. Given that the putative causative agent is a new strain of coronavirus that has not previously affected humans, it will be critical to obtain more information on the in vitro susceptibility of this virus to ribavirin and other investigational and licensed antivirals. The US Food and Drug Administration has initiated such a screening program.
Until more information becomes available on the efficacy of ribavirin and the optimal management of SARS, it is likely that use will continue to be recommended at least in a subset of sicker patients. Canadian physicians should become familiar with the contradictions to ribavirin use and the established adverse effects of the drug and should closely monitoring patients receiving ribavirin for as-yet-undescribed short-term and long-term adverse events.
β See related article pages 1245, 1259, 1265, 1294
Supplementary Material
Figure.

Acknowledgments
Gideon Koren is a Senior Scientist of the Canadian Institutes of Health Research. Elizabeth Phillips is a Career Scholar of the Ontario HIV Treatment Network.
Footnotes
This paper is sponsored by the Canadian Society for Clinical Pharmacology.
Fast-tracked article, published at www.cmaj.ca on Apr. 16, 2003
Contributors: All authors participated in writing the article. Gideon Koren coordinated the review process and reviewed the sections on pediatric use of ribavirin and use of the drug in pregnancy. Susan King wrote and reviewed the section on pediatric use, as well as the section on indications. Sandra Knowles and Elizabeth Phillips reviewed the sections on adverse effects and the SARS experience in Toronto; Elizabeth Phillips also reviewed the guidelines for adult use of the drug.
Correspondence to: Dr. Gideon Koren, Division of Clinical Pharmacology/Toxicology, The Hospital for Sick Children, 555 University Ave., Toronto ON M5G 1X8; fax 416 813-7562; gkoren@sickkids.ca
References
- 1.Cameron CE, Castro C. The mechanism of action of ribavirin: lethal mutagenesis of RNA virus genomes mediated by the viral RNA-dependent RNA polymerase. Curr Opin Infect Dis 2001;14:757-64. [DOI] [PubMed]
- 2.Glue P. The clinical pharmacology of ribavirin. Semin Liver Dis 1999;19:17-24. [PubMed]
- 3.Smith DW, Frankel LR, Mathers LH, Tang AT, Arigno RL, Prober CG. A controlled trial of aerosolized ribavirin in infants receiving mechanical ventilation for severe respiratory syncytial virus infection. N Engl J Med 1991; 325:24-9. [DOI] [PubMed]
- 4.Huggins JW, Hsiang CM, Cosgriff TM, Guang MY, Smith JI, Wu ZO, et al. Prospective, double blind, concurrent, placebo-controlled clinical trial of intravenous ribavirin therapy of hemorrhagic fever with renal syndrome. J Infect Dis 1991;164:1119-27. [DOI] [PubMed]
- 5.National Institutes of Health consensus development conference statement. Management of hepatitis C: 2002. Bethesda (MD): National Institutes of Health; 2002. www.consensus.nih.gov/cons/116/Hepc091202.pdf (accessed 2003 Apr 15).
- 6.Chang CH, Chen KY, Lai MY, Chan KA. Meta-analysis: ribavirin-induced hemolytic anaemia in patients with chronic hepatitis C. Aliment Pharmacol Ther 2002;16:1623-32. [DOI] [PubMed]
- 7.Lafeuillade A, Hittinger G, Chadapaud S. Increased mitochondrial toxicity with ribavirin in HIV/HCV coinfection. Lancet 2001;357:280-1. [DOI] [PubMed]
- 8.Pockros PJ, Reindollar R, McHutchinson J, Reddy R, Wright T, Boyd DG, et al. The safety and tolerability of daily infergen plus ribavirin in the treatment of naïve chronic hepatitis C patients. J Viral Hepat 2003;10:55-60. [DOI] [PubMed]
- 9.Bertrand P, Faro A, Cantwell P, Tzakis A. Intravenous ribavirin and hyperammonemia in an immunocompromised patient infected with adenovirus. Pharmacotherapy 2000;20:1296. [DOI] [PubMed]
- 10.Chapman LE, Mertz GJ, Peters CJ, Jolson HM, Khan AS, Ksiazek TG, et al. Intravenous ribavirin for hantavirus pulmonary syndrome: safety and tolerance during 1 year of open-label experience. Ribavirin Study Group. Antivir Ther 1999;4:211-9. [DOI] [PubMed]
- 11.Chutaputti A. Management of hepatitis C: adverse effects and other safety aspects of the hepatitis C antivirals. J Gastroenterol Hepatol 2000; 15 (Suppl): E156-63. [DOI] [PubMed]
- 12.Schulman S. Inhibition of warfarin activity by ribavirin. Ann Pharmacother 2002;36:72-4. [DOI] [PubMed]
- 13.Infectious Diseases and Immunization Committee, Canadian Paediatric Society. Ribavirin: Is there a risk to hospital personnel? [position statement]. CMAJ 1991;144(3):285-6. [PMC free article] [PubMed]
- 14.Ito S, Koren G. Exposure of pregnant women to ribavirin-contaminated air: risk assessment and recommendations. Pediatr Infect Dis J 1993;12:2-5. [DOI] [PubMed]
- 15.Office of Women's Health. Promoting healthy pregnancies [Web page]. Rockville (MD): US Food and Drug Administration; 2003. Available: www.fda.gov/womens/registries/ (accessed 2003 Apr 16).
- 16.Poutanen SM, Low DE, Henry B, Finkelstein S, Rose D, Green K, et al. Identification of severe acute respiratory syndrome in Canada [online]. N Engl J Med. Available: content.nejm.org/cgi/reprint/NEJMoa030634v3 (posted 2003 Mar 31; accessed 2003 Apr 15). [DOI] [PubMed]
- 17.Tsang KW, Ho PL, Ooi GC, Yee WK, Wang T, Chan-Yeung M, et al. A cluster of cases of severe acute respiratory syndrome in Hong Kong [online]. N Engl J Med. Available: content.nejm.org/cgi/reprint/NEJMoa030666v3 (posted 2003 Mar 31; accessed 2003 Apr 15).
- 18.Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt GM, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong [online]. N Engl J Med. Available: content.nejm.org/cgi/reprint/NEJMoa030685v2 (posted 2003 Apr 7; accessed 2003 Apr 15). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


