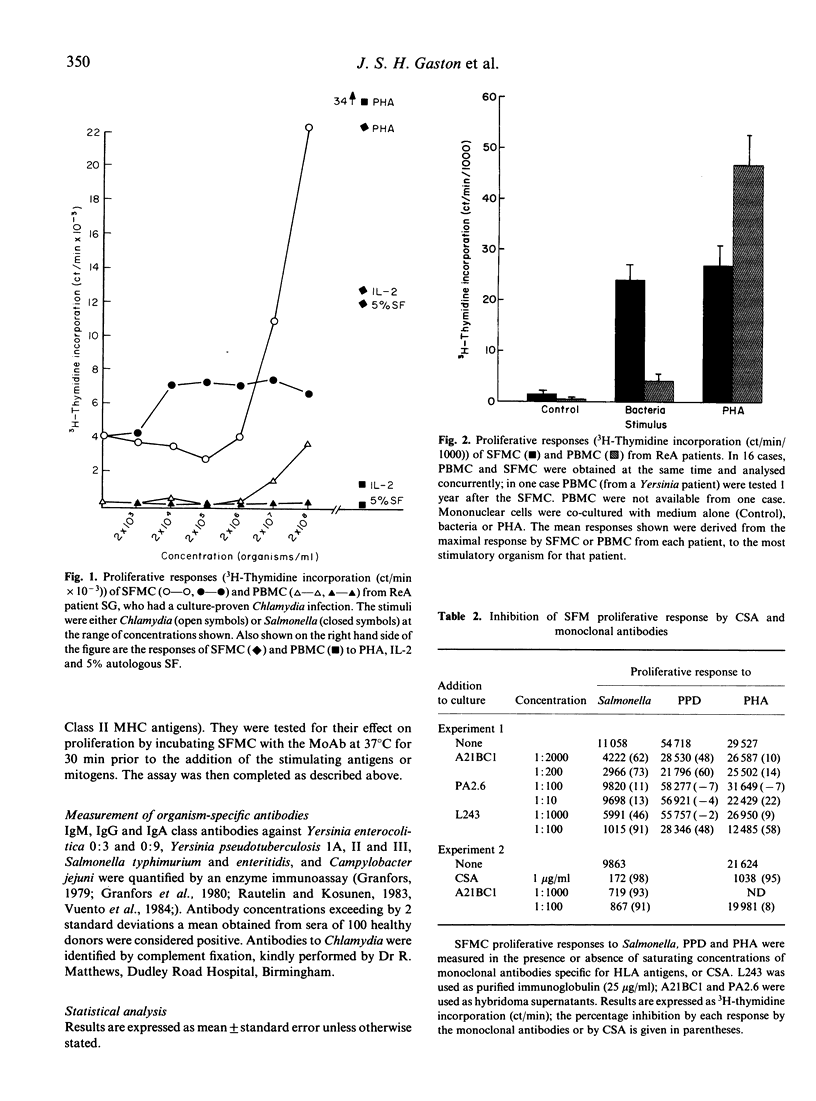
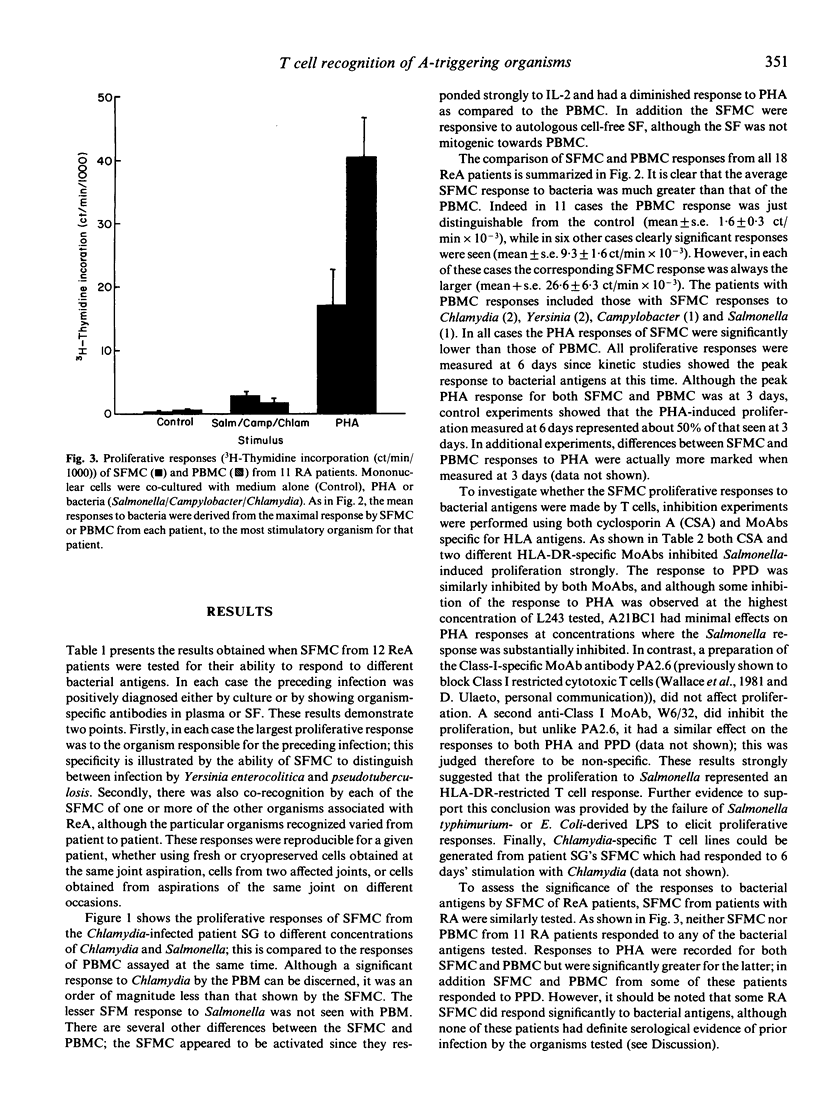
Abstract
Reactive arthritis (ReA) is believed to be "triggered' by infection with certain bacteria. When the proliferative responses of mononuclear cells (MC) obtained from the synovial fluid (SF) of ReA patients were examined, it was found that they responded maximally to the specific organism responsible for the preceding infection. The response was shown to be due to Class II MHC-restricted T cells by inhibition experiments using cyclosporin A and monoclonal antibodies. Significant SFMC responses to additional organisms associated with ReA were also recorded; since there was no serological evidence of preceding infection by these organisms, this finding suggests that these bacteria share common T cell-recognized antigenic epitopes. The corresponding responses by peripheral blood mononuclear cells (PBMC) were much lower and often barely detectable, whereas their responses to PHA were consistently higher than those of SFMC. These results, combined with evidence that bacterial antigens localize in the joint, indicate that a bacteria-specific, T-cell-mediated response may play a central role in the pathogenesis of ReA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aho K., Leirisalo-Repo M., Repo H. Reactive arthritis. Clin Rheum Dis. 1985 Apr;11(1):25–40. [PubMed] [Google Scholar]
- Akbar A. N., Terry L., Timms A., Beverley P. C., Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988 Apr 1;140(7):2171–2178. [PubMed] [Google Scholar]
- Brenner M. B., Kobayashi S., Wiesenhutter C. W., Huberman A. K., Bales P., Yu D. T. In vitro T lymphocyte proliferative response to Yersinia enterocolitica in Reiter's syndrome. Lack of response in other HLA-B27 positive individuals. Arthritis Rheum. 1984 Mar;27(3):250–257. doi: 10.1002/art.1780270302. [DOI] [PubMed] [Google Scholar]
- Brewerton D. A., Caffrey M., Nicholls A., Walters D., Oates J. K., James D. C. Reiter's disease and HL-A 27. Lancet. 1973 Nov 3;302(7836):996–998. doi: 10.1016/s0140-6736(73)91091-x. [DOI] [PubMed] [Google Scholar]
- Emery P., Gentry K. C., Mackay I. R., Muirden K. D., Rowley M. Deficiency of the suppressor inducer subset of T lymphocytes in rheumatoid arthritis. Arthritis Rheum. 1987 Aug;30(8):849–856. doi: 10.1002/art.1780300802. [DOI] [PubMed] [Google Scholar]
- Ford D. K. Reactive arthritis: a viewpoint rather than a review. Clin Rheum Dis. 1986 Aug;12(2):389–401. [PubMed] [Google Scholar]
- Ford D. K., Reid G. D., Magge S., Schumacher H. R. Synovial lymphocyte response to chlamydial stimulation associated with intrasynovial chlamydial antigen in a patient with "rheumatoid arthritis". Arthritis Rheum. 1988 Jul;31(7):914–917. doi: 10.1002/art.1780310714. [DOI] [PubMed] [Google Scholar]
- Ford D. K., da Roza D. M., Schulzer M. The specificity of synovial mononuclear cell responses to microbiological antigens in Reiter's syndrome. J Rheumatol. 1982 Jul-Aug;9(4):561–567. [PubMed] [Google Scholar]
- Ford D. K., da Roza D. M., Ward R. H. Arthritis confined to knee joints. Synovial lymphocyte responses to microbial antigens correlate with distribution of HLA. Arthritis Rheum. 1984 Oct;27(10):1157–1164. doi: 10.1002/art.1780271012. [DOI] [PubMed] [Google Scholar]
- Ford D. K., da Roza D., Schulzer M. Lymphocytes from the site of disease but not blood lymphocytes indicate the cause of arthritis. Ann Rheum Dis. 1985 Oct;44(10):701–710. doi: 10.1136/ard.44.10.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granfors K., Jalkanen S., von Essen R., Lahesmaa-Rantala R., Isomäki O., Pekkola-Heino K., Merilahti-Palo R., Saario R., Isomäki H., Toivanen A. Yersinia antigens in synovial-fluid cells from patients with reactive arthritis. N Engl J Med. 1989 Jan 26;320(4):216–221. doi: 10.1056/NEJM198901263200404. [DOI] [PubMed] [Google Scholar]
- Granfors K. Measurement of immunoglobulin M (IgM), IgG, and IgA antibodies against Yersinia enterocolitica by enzyme-linked immunosorbent assay: persistence of serum antibodies during disease. J Clin Microbiol. 1979 Mar;9(3):336–341. doi: 10.1128/jcm.9.3.336-341.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granfors K., Toivanen A. IgA-anti-yersinia antibodies in yersinia triggered reactive arthritis. Ann Rheum Dis. 1986 Jul;45(7):561–565. doi: 10.1136/ard.45.7.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granfors K., Viljanen M., Tiilikainen A., Toivanen A. Persistence of IgM, IgG, and IgA antibodies to Yersinia in yersinia arthritis. J Infect Dis. 1980 Apr;141(4):424–429. doi: 10.1093/infdis/141.4.424. [DOI] [PubMed] [Google Scholar]
- Keat A., Thomas B., Dixey J., Osborn M., Sonnex C., Taylor-Robinson D. Chlamydia trachomatis and reactive arthritis: the missing link. Lancet. 1987 Jan 10;1(8524):72–74. doi: 10.1016/s0140-6736(87)91910-6. [DOI] [PubMed] [Google Scholar]
- Lahesmaa-Rantala R., Granfors K., Isomäki H., Toivanen A. Yersinia specific immune complexes in the synovial fluid of patients with yersinia triggered reactive arthritis. Ann Rheum Dis. 1987 Jul;46(7):510–514. doi: 10.1136/ard.46.7.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindblad S., Klareskog L., Hedfors E., Forsum U., Sundström C. Phenotypic characterization of synovial tissue cells in situ in different types of synovitis. Arthritis Rheum. 1983 Nov;26(11):1321–1332. doi: 10.1002/art.1780261104. [DOI] [PubMed] [Google Scholar]
- ROPES M. W., BENNETT G. A., COBB S., JACOX R., JESSAR R. A. 1958 Revision of diagnostic criteria for rheumatoid arthritis. Bull Rheum Dis. 1958 Dec;9(4):175–176. [PubMed] [Google Scholar]
- Rautelin H., Kosunen T. U. An acid extract as a common antigen in Campylobacter coli and Campylobacter jejuni strains. J Clin Microbiol. 1983 Apr;17(4):700–701. doi: 10.1128/jcm.17.4.700-701.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon M., Kitas G. D., Gaston J. S., Bacon P. A. Interleukin-2 production and response by helper T-cell subsets in man. Immunology. 1988 Sep;65(1):81–85. [PMC free article] [PubMed] [Google Scholar]
- Schumacher H. R., Jr, Magge S., Cherian P. V., Sleckman J., Rothfuss S., Clayburne G., Sieck M. Light and electron microscopic studies on the synovial membrane in Reiter's syndrome. Immunocytochemical identification of chlamydial antigen in patients with early disease. Arthritis Rheum. 1988 Aug;31(8):937–946. doi: 10.1002/art.1780310801. [DOI] [PubMed] [Google Scholar]
- Teyton L., Lotteau V., Turmel P., Arenzana-Seisdedos F., Virelizier J. L., Pujol J. P., Loyau G., Piatier-Tonneau D., Auffray C., Charron D. J. HLA DR, DQ, and DP antigen expression in rheumatoid synovial cells: a biochemical and quantitative study. J Immunol. 1987 Mar 15;138(6):1730–1738. [PubMed] [Google Scholar]
- Toivanen A., Granfors K., Lahesmaa-Rantala R., Leino R., Ståhlberg T., Vuento R. Pathogenesis of Yersinia-triggered reactive arthritis: immunological, microbiological and clinical aspects. Immunol Rev. 1985 Aug;86:47–70. doi: 10.1111/j.1600-065x.1985.tb01137.x. [DOI] [PubMed] [Google Scholar]
- Toivanen A., Lahesmaa-Rantala R., Vuento R., Granfors K. Association of persisting IgA response with yersinia triggered reactive arthritis: a study on 104 patients. Ann Rheum Dis. 1987 Dec;46(12):898–901. doi: 10.1136/ard.46.12.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuento R., Leino R., Viander M., Toivanen A. In vitro lymphoproliferative response to Yersinia: depressed response in arthritic patients years after Yersinia infection. Clin Exp Rheumatol. 1983 Jul-Sep;1(3):219–224. [PubMed] [Google Scholar]
- Wallace L. E., Moss D. J., Rickinson A. B., McMichael A. J., Epstein M. A. Cytotoxic T cell recognition of Epstein-Barr virus-infected B cells. II. Blocking studies with monoclonal antibodies to HLA determinants. Eur J Immunol. 1981 Sep;11(9):694–699. doi: 10.1002/eji.1830110905. [DOI] [PubMed] [Google Scholar]
- Wang A., Lu S. D., Mark D. F. Site-specific mutagenesis of the human interleukin-2 gene: structure-function analysis of the cysteine residues. Science. 1984 Jun 29;224(4656):1431–1433. doi: 10.1126/science.6427925. [DOI] [PubMed] [Google Scholar]


